Read more:
Our most commonly queried extra core features are below.
Looking for something else? See our full list of core features here.
Solutions
Looking for something more specific? Read through the other solutions we support out of the box
Batch Records
Utilize Batch Records to prevent errors and negative impacts to the final product
Environmental Monitoring
Unify and simplify your environmental monitoring workflows by centralizing data management
Stability
Reliably manage your stability programs, reducing errors, and providing a toolset for effective stability testing, analysis, and reporting
Utilize Batch Records to prevent errors and negative impacts to the final product
Environmental Monitoring
Unify and simplify your environmental monitoring workflows by centralizing data management
Stability
Reliably manage your stability programs, reducing errors, and providing a toolset for effective stability testing, analysis, and reporting
Next Generation Sequencing
Ensure secure management and tracking of NGS samples from extraction to data analysis
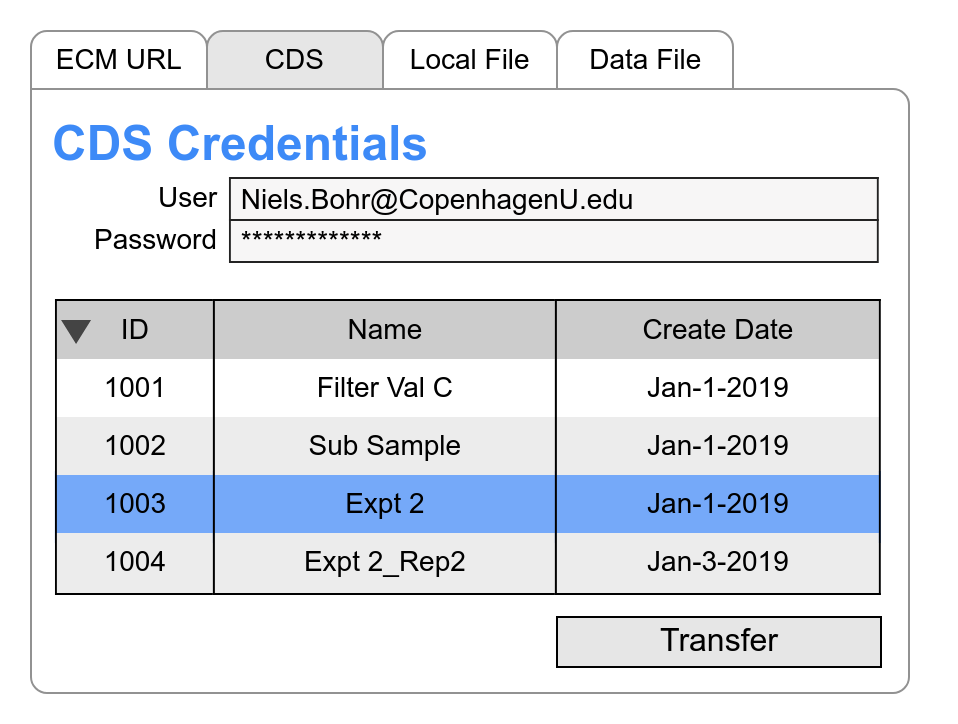
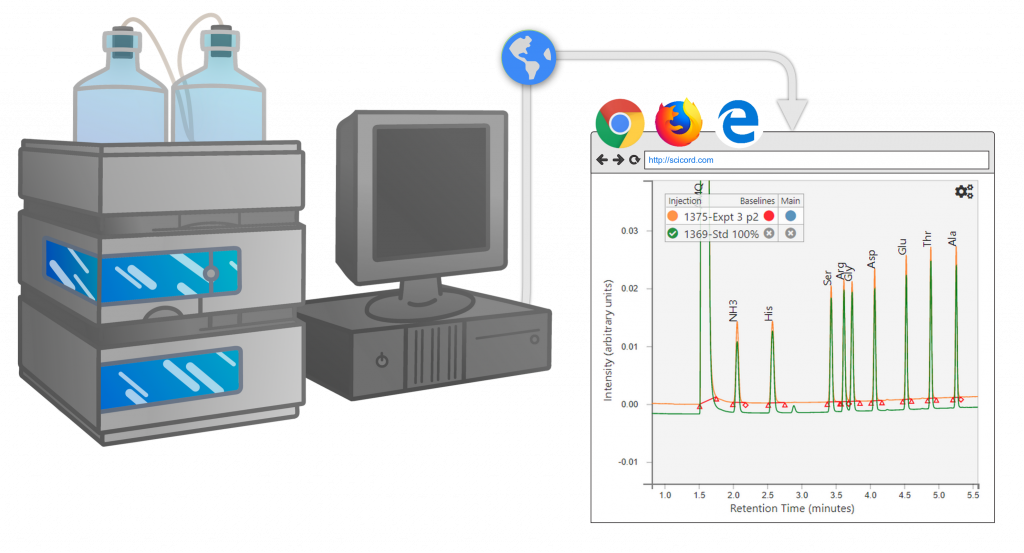
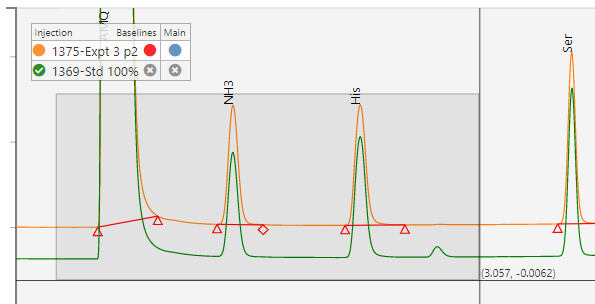
Chromatography
Expose your chromatography data across your organization to improve decision making
Mass Spec
Elevate efficiency, assure compliance, and streamline your Mass Spec information process
Ensure secure management and tracking of NGS samples from extraction to data analysis
Chromatography
Expose your chromatography data across your organization to improve decision making
Mass Spec
Elevate efficiency, assure compliance, and streamline your Mass Spec information process
Resources
Browse our archive case studies, functional documentation, and announcements.
Looking for something specific? Search our site:
See case studies on implementations our customers have used to achieve specific outcomes:
Other Resources:
Frequently used or consulted tools and information
Our Documentation:
Search these pages for a wider variety of documentation