4. Test Results
Capture results with precision while enforcing roles, specifications, and real-time validation
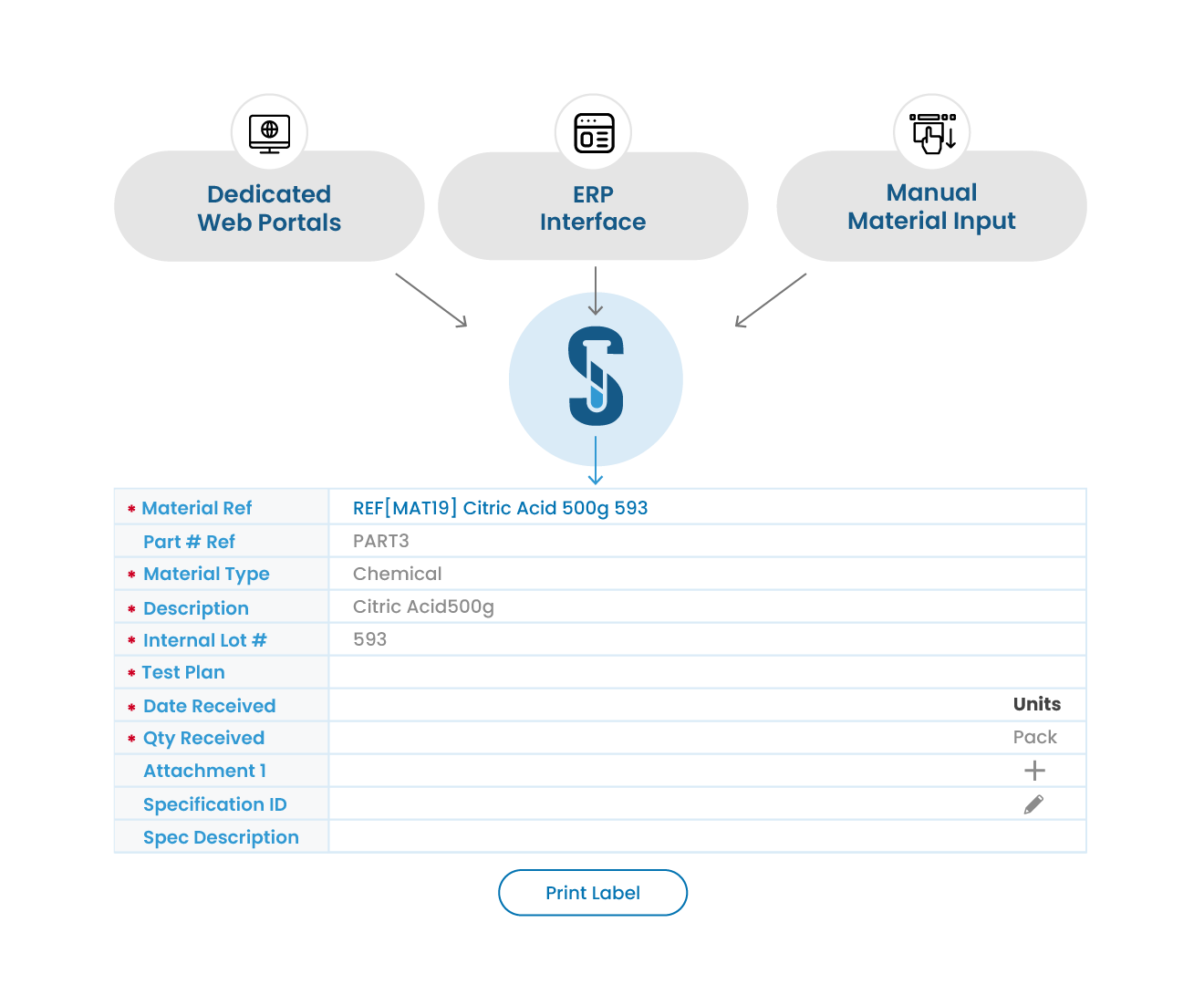
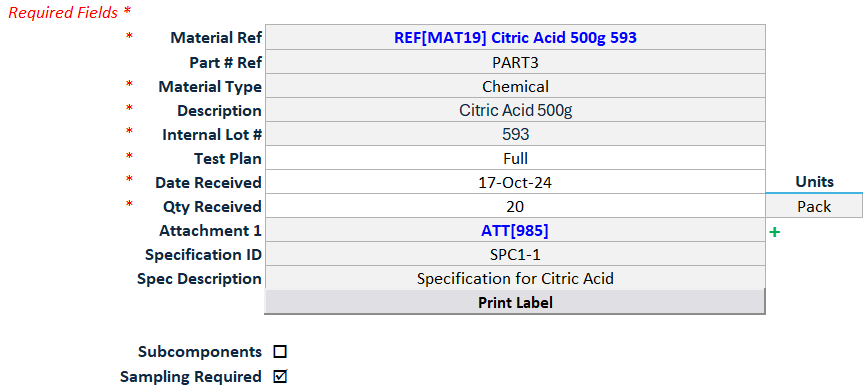
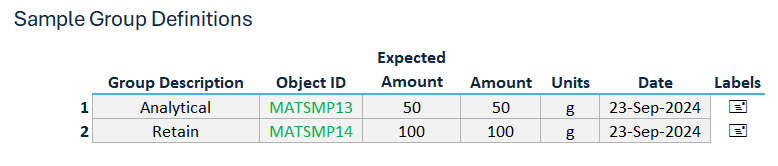
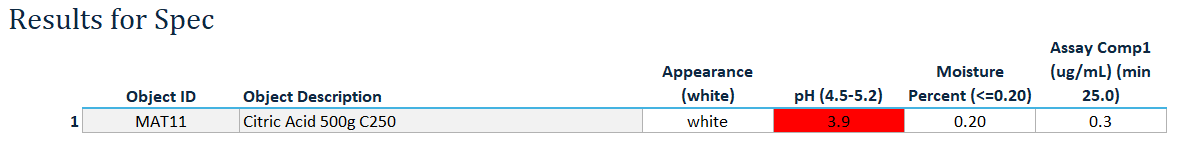
Linked Result Recorder: Sample groups delivered to the lab are linked to dedicated ‘result recorder’ worksheets and associated with the appropriate specifications, based on the defined Sampling Plan and Inspection Record, ensuring only the correct tests are populated for result entry.
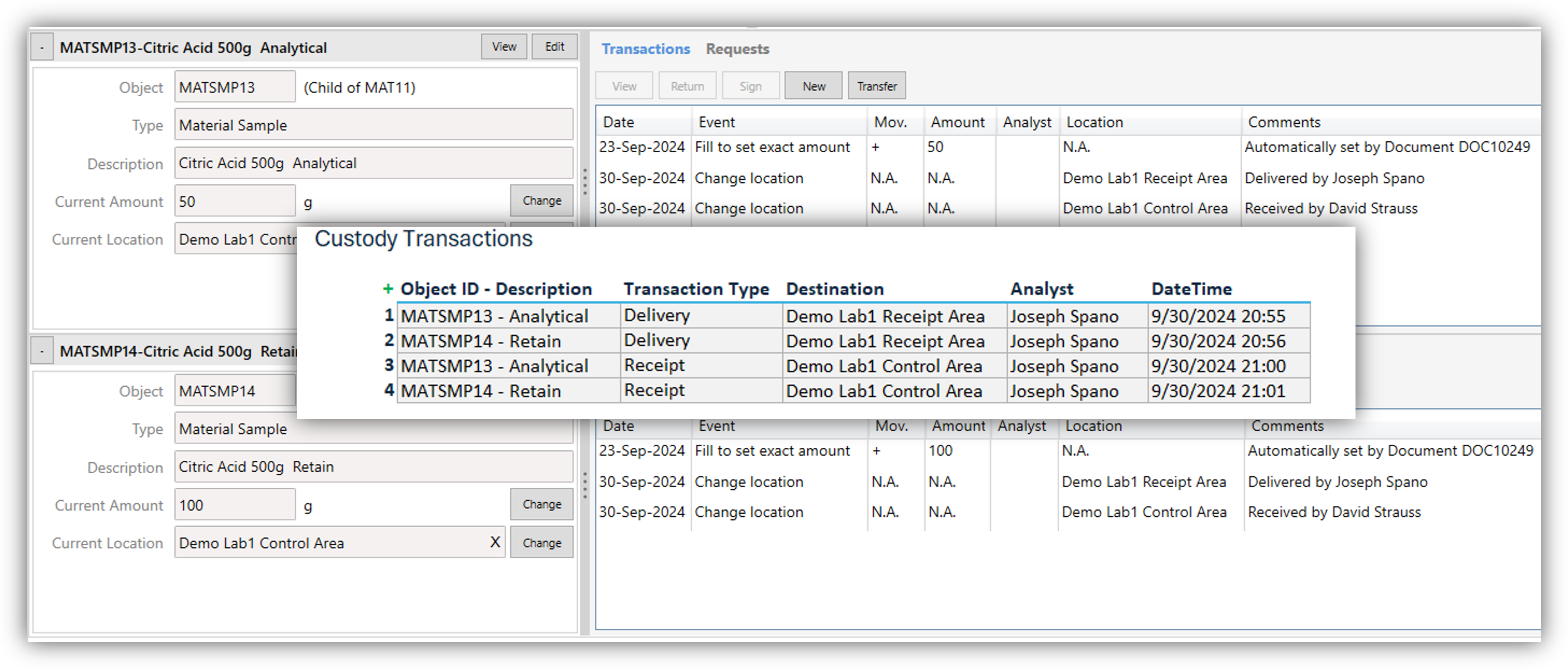
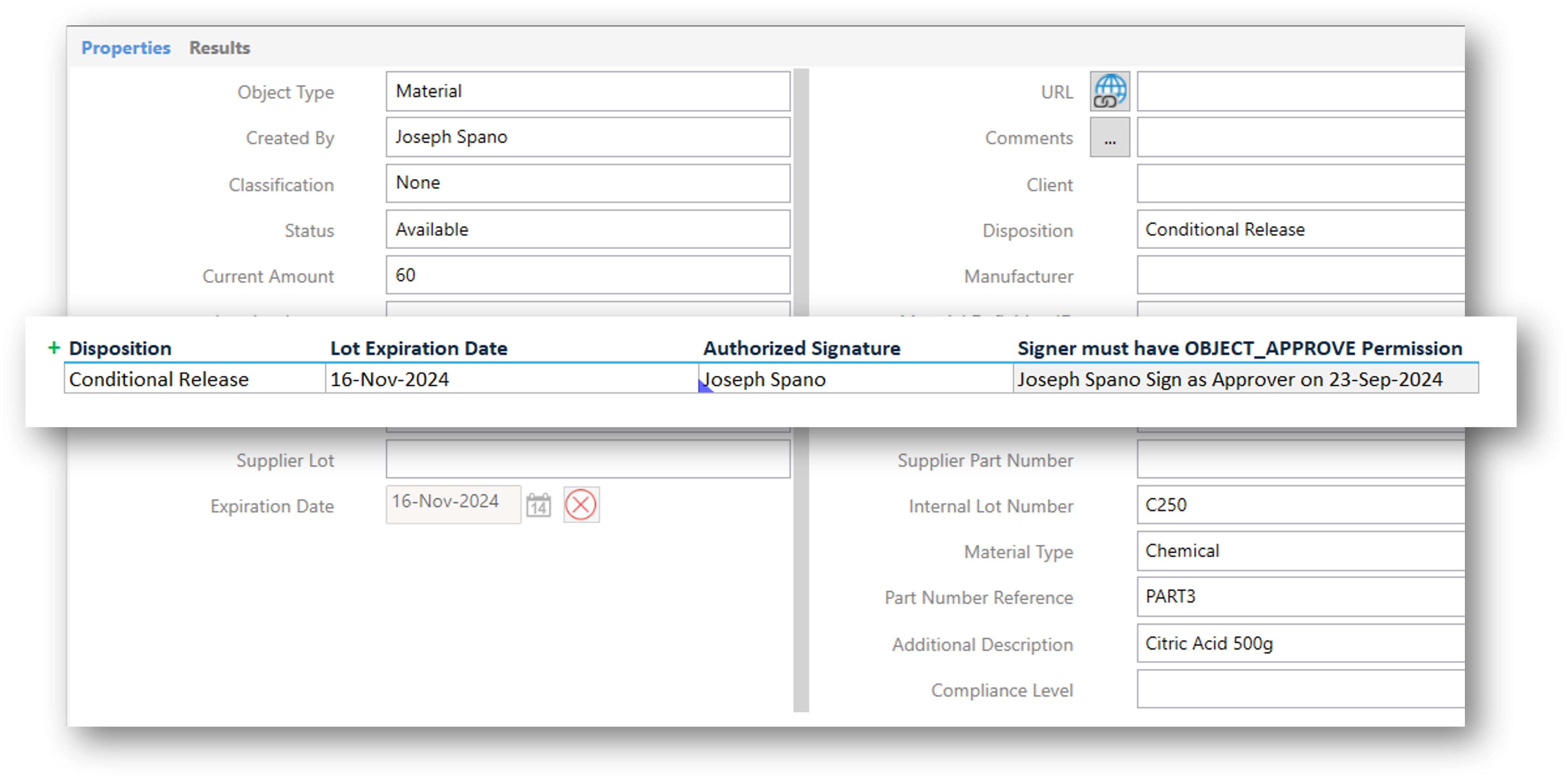
Role-Based Access Control: Enforce roles and access levels where analysts can enter and authorize results, reviewers can authorize tests, and supervisors can authorize samples.
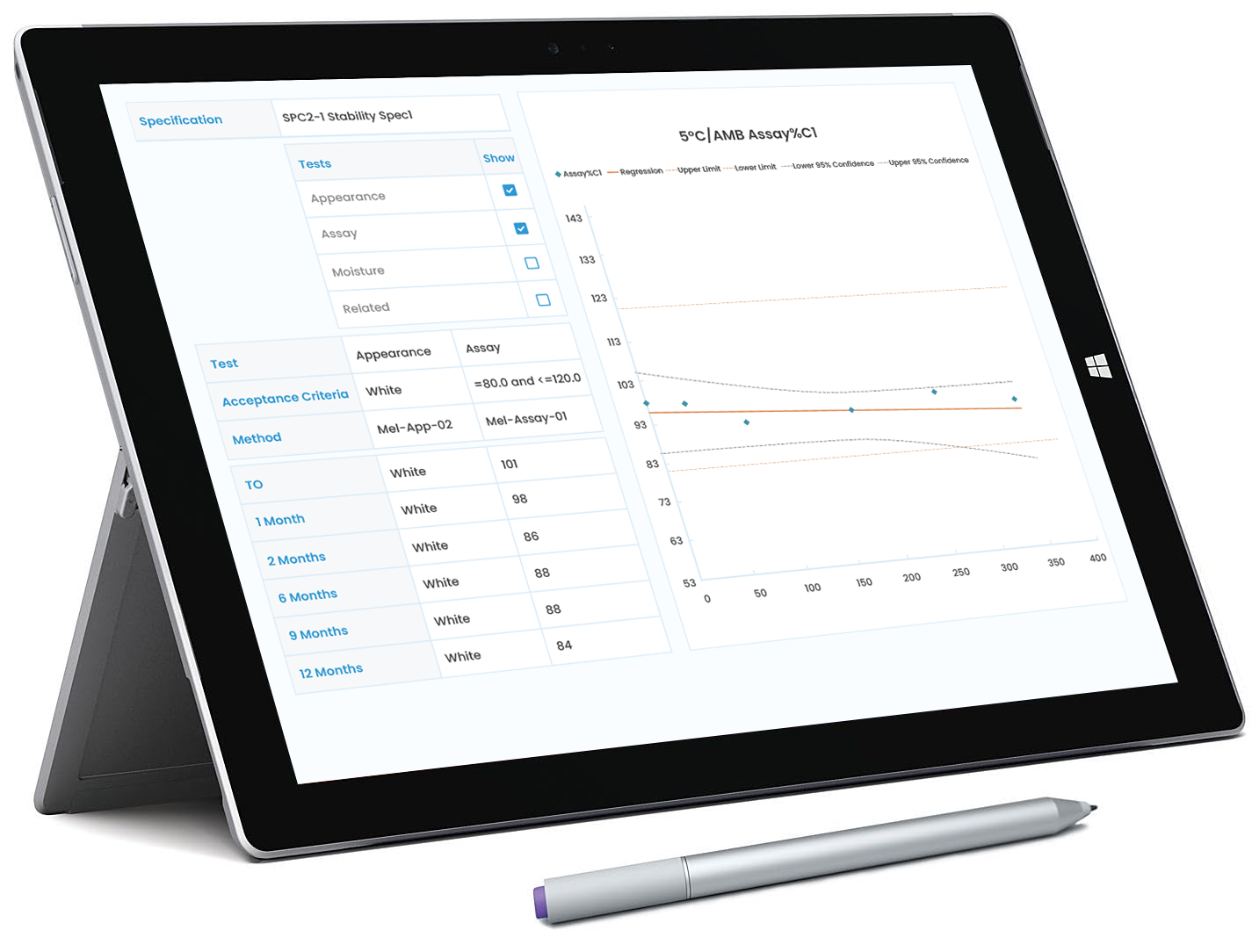
Flexible Test Entry with Validation: Input test results in multiple formats (numeric, text, or CSV import) with real-time validation and automatic flagging, triggering investigation prompts for any out-of-spec results.