Equipment Logbook Entries
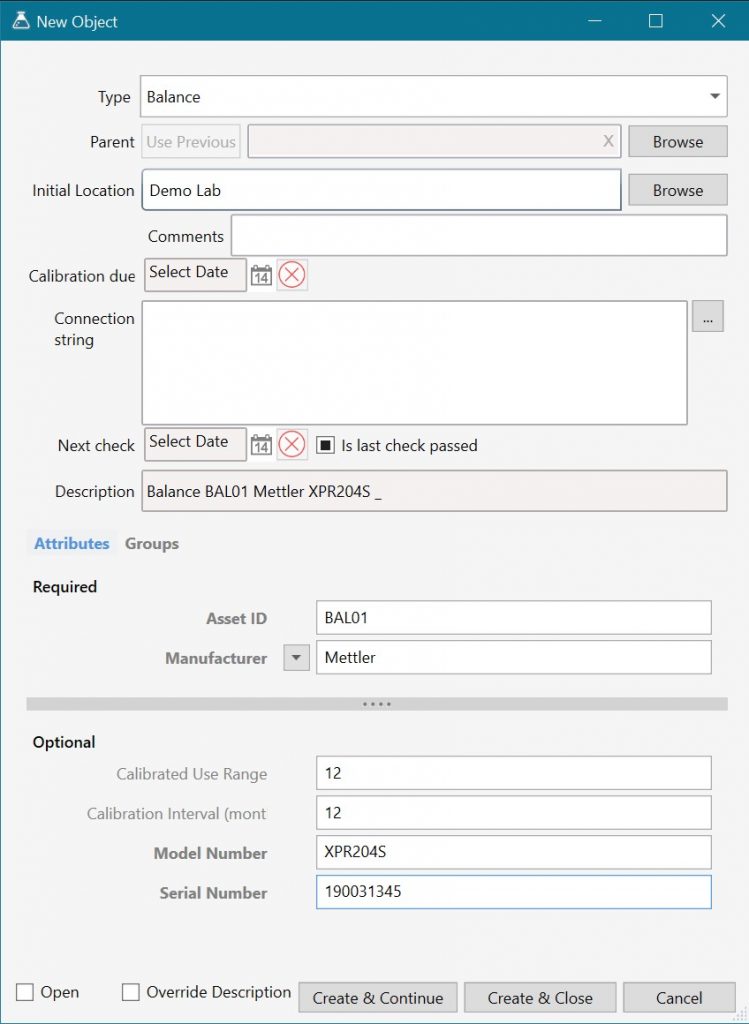
Equipment can be quickly logged into the system using our predefined equipment types – such as balance, pH meter, etc. These types can be customized or expanded with additional types that are relevant for your lab and capture all of the vital information.
Each type has a predefined set of attributes for things like Asset ID or calibration info. These can be changed based on what data the lab needs to store, to do additional things like keep links to third party asset management systems.
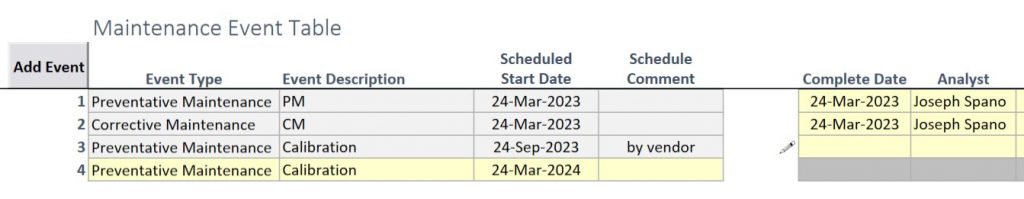
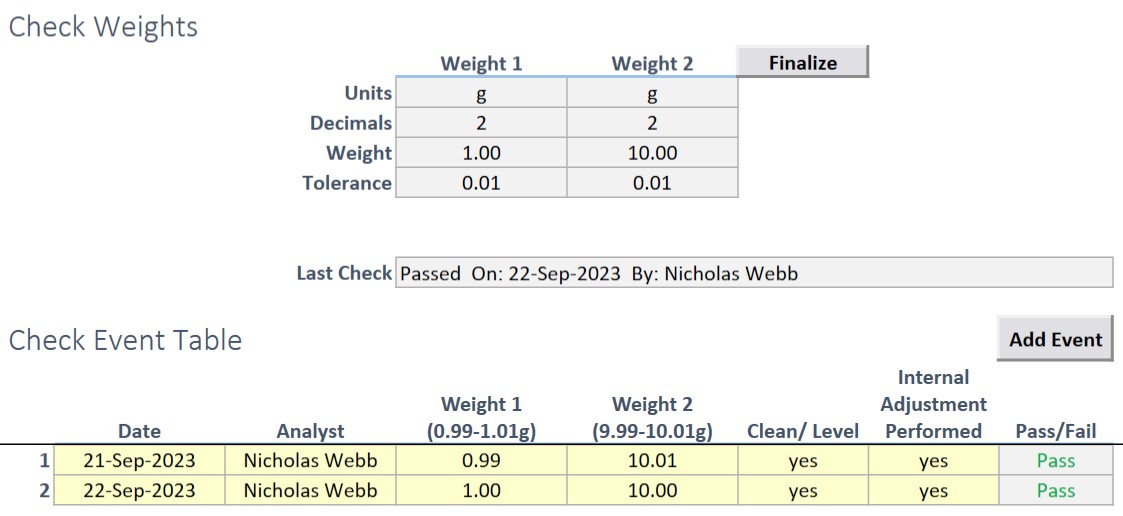
All equipment types also have access to the connection string module (for connecting to IP or Serial instruments and directly reading data) and the Next check module for ensuring that the equipment is not used if there is a pending check (such as for daily balance checks). Direct connection ensures that accurate data is transferred from the piece of equipment to the appropriate location in a workbook.