Right First Time
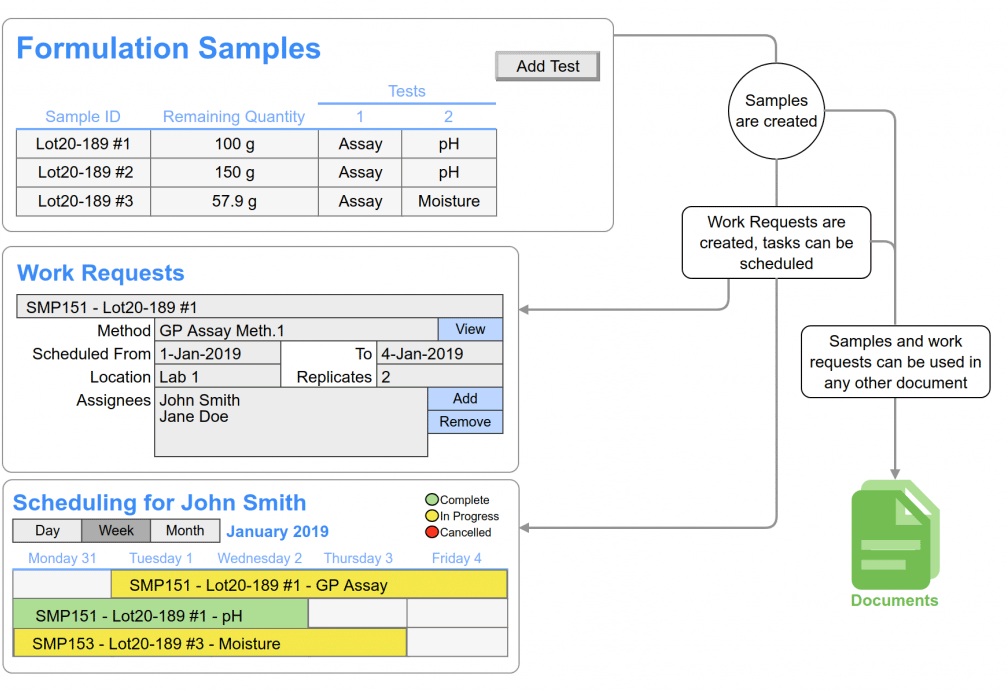
- Work process templates, guide and inform the required process steps and assure comprehensive documentation.
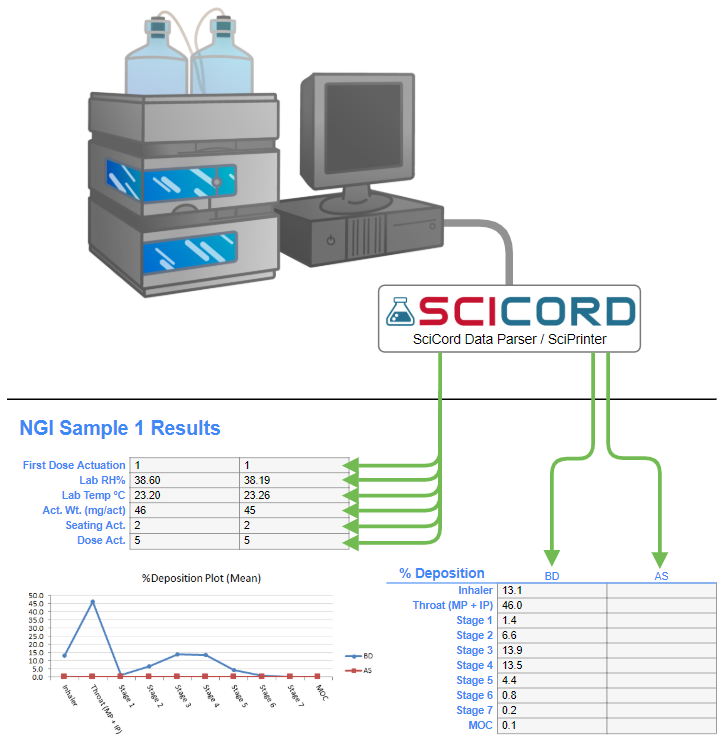
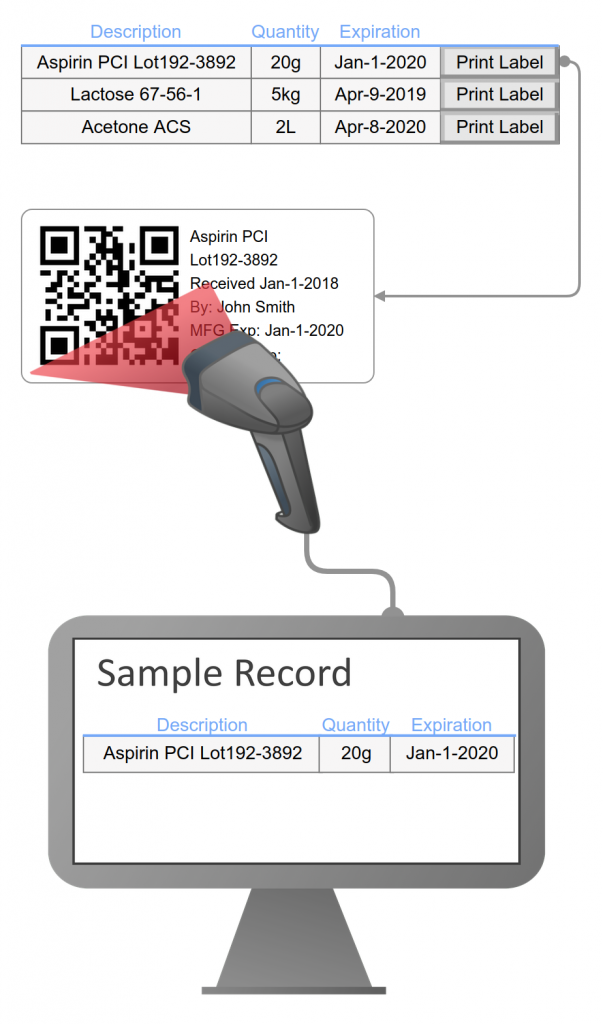
- Interfaced instruments to eliminate transcription errors
- Automated/validated calculations generate accurate reportable data, managing the required precision, and automatically updating when a contributing data point is recorded/corrected.
Compliance
- 21 CFR part 11 compliant, validated, and version-controlled spreadsheet templates.
- Visual audit trail to easily track your notebook records and eliminate regulatory worries.
- Electronic signatures for accountability and lifecycle decisions.
- Optimized review & approval process increases productivity by 30%.
- Your data is secure in Microsoft Azure and with cloud security and automatic backups.
Decision Making
- Visualized data lets analysts see the variability generated by changes in flow rates, spray patterns, through use, sample preps, test conditions, data transcription, and analyst techniques.
- Visualization highlights anomalies in the analytical data, reduces the amount of effort required to identify their sources, and supports enhanced quality.
- Address the unique difficulties encountered by labs testing DMDIs and DPIs and provide an efficient means to pinpoint anomalies, identify their sources, and provide better QC.
Specialized Inhalation Templates
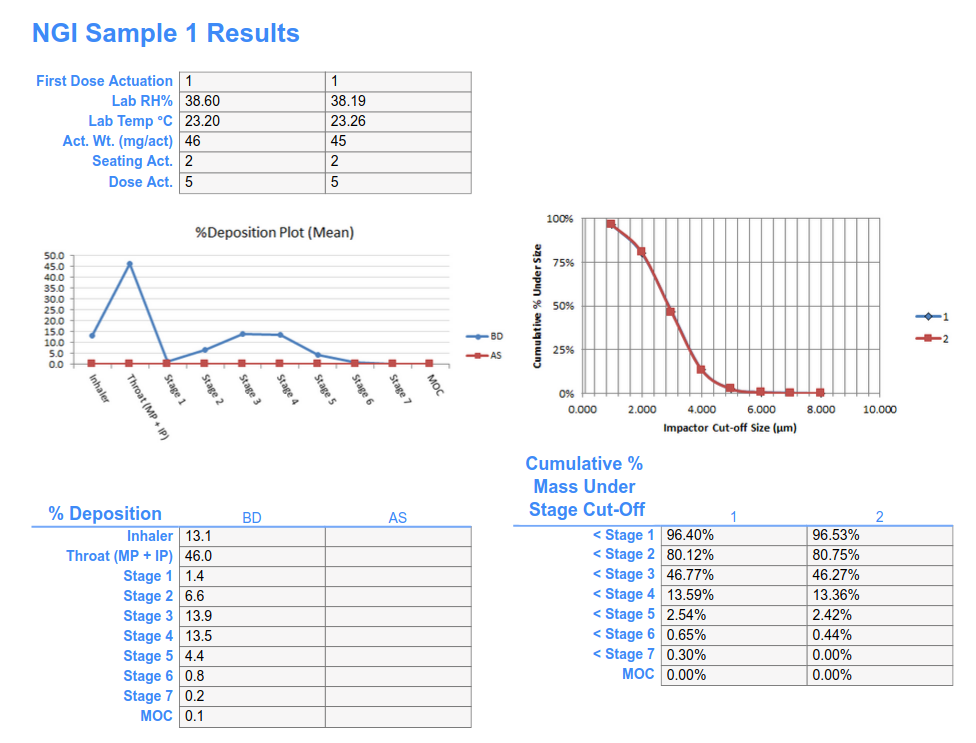
- Off-the-shelf templates are available for: Aerodynamic Particle Size Distribution, Delivered Dose Uniformity, Water Content, Leakage, Number of Discharges, and more. Additional templates can be created by your team or in partnership with SciCord engineers.
- Validated processes and calculations generate data you can be confident in.
- Information recorded in the templates is automatically converted into structured data for visualizations & reporting
- Includes interface with instruments (balance, chromatography system, moisture meter, pH meter…) to generate run sequences, record instrument reports, and parse measurement data.
- Manages complex sample preparations to increase measurement accuracy, reduce errors of transposition or substitution, with references to equipment, instruments, and reference standards, standards, and solutions.
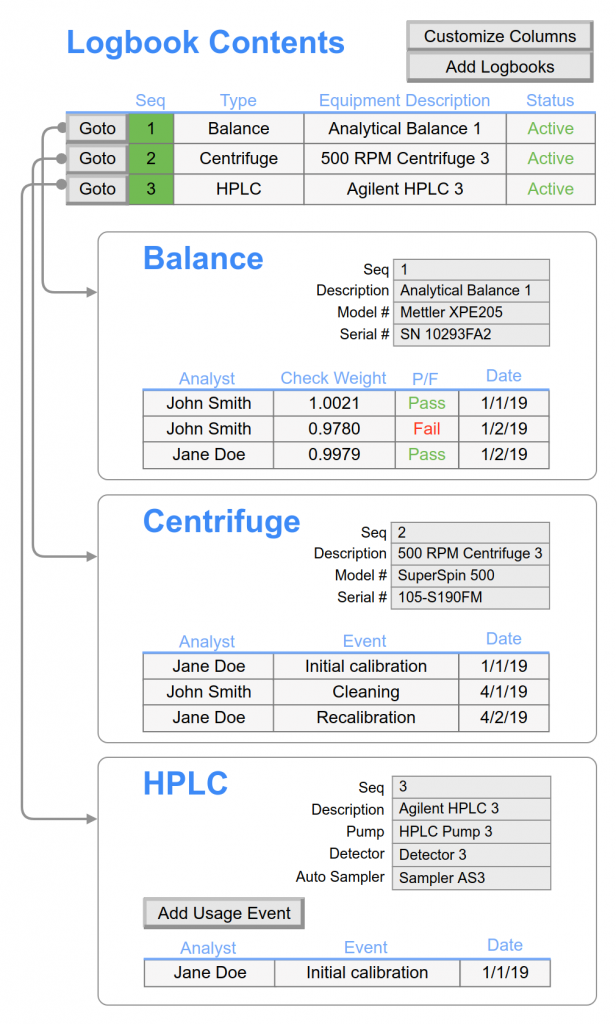
Equipment & Instrument Management
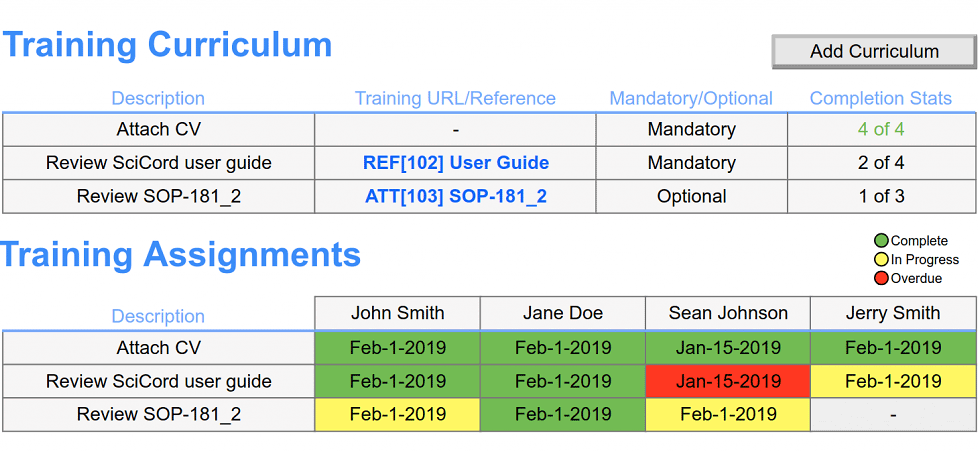
- Electronically document equipment/instrument inventory.
- Schedule calibration or maintenance events.
- Record the details and attach vendor documentation for completed calibration or maintenance events.
- Record daily instrument checks
- Manage instrument interface parameters
- Automatically log equipment use for usage reports or quickly identify instruments in investigations.
Review
Optimized review process increases productivity by 30% over a paper based process.
Spreadsheet Paradigm
- Off-the-shelf templates are available for: Aerodynamic Particle Size Distribution, Delivered Dose Uniformity, Water Content, Leakage, Number of Discharges, and more. Additional templates can be created by your team or in partnership with SciCord engineers.
- Validated processes and calculations generate data you can be confident in.
- Information recorded in the templates is automatically converted into structured data for visualizations & reporting
DataMart
Your process and analytical information collated in data tables specifically designed for the inhaled development team. SQL queries support easily integration with your existing statistical packages or visualization tools (Microsoft Excel®, Microsoft Power BI®, Tibco Spotfire®, SAS JMP®, etc.) for analysis and trending.
21 CFR
- Validated, and version-controlled spreadsheet templates
- Visual audit trail captures who did what, why, and when.
- Electronic signatures for workflow and key process decisions.
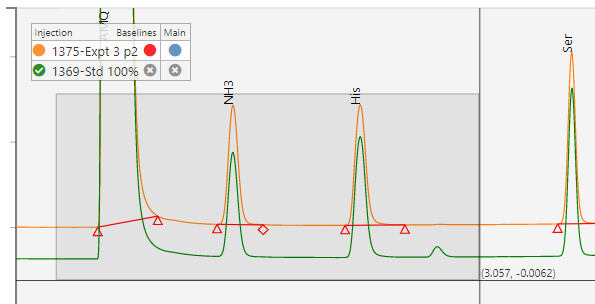
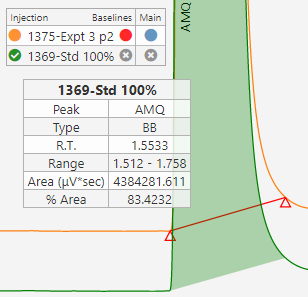
Sequence Visualization
A browser based sequence viewer eliminates the need for reviewers, approvers and decision makers to access & navigate the chromatography system.
A simple, demo version of SciCord’s SciChrom Viewer is accessible below. A guide is also available if you need more information about how SciChrom Viewer works.
SciChrom Viewer is meant to be used on a full desktop/laptop (not phone/tablet) and on the Chrome, Edge, or Firefox browsers. Please use one of these devices/browsers.
You can also download the user guide if you want to get an idea of how SciChrom Viewer works but don’t have access to a computer.
If you want a more technical discussion about inhalation in general, you can
read more here.