Dashboard
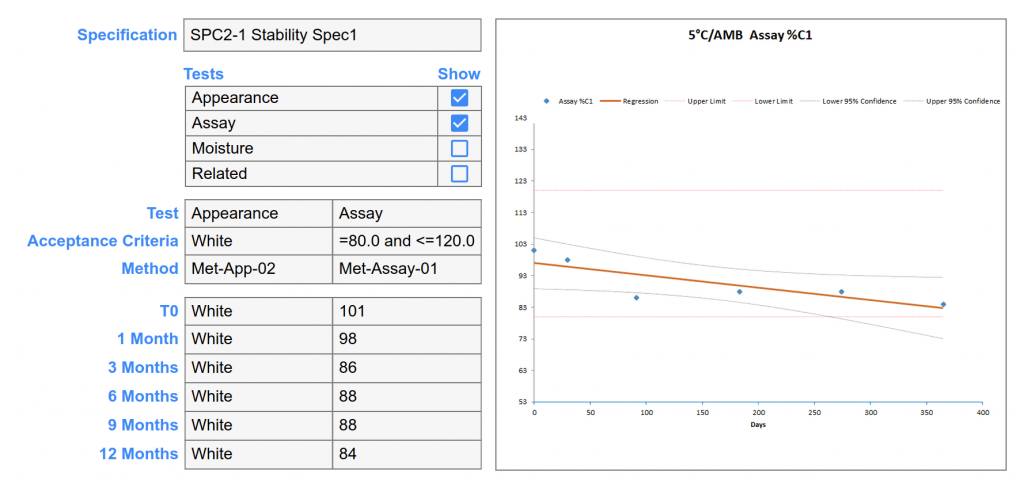
- Visual display for real-time monitoring of stability studies, including status, test completion, and deviations.
- Automated alerts and notifications for scheduled inventory transaction, scheduled tests, deviations, and out-of-specification results
Sample Management
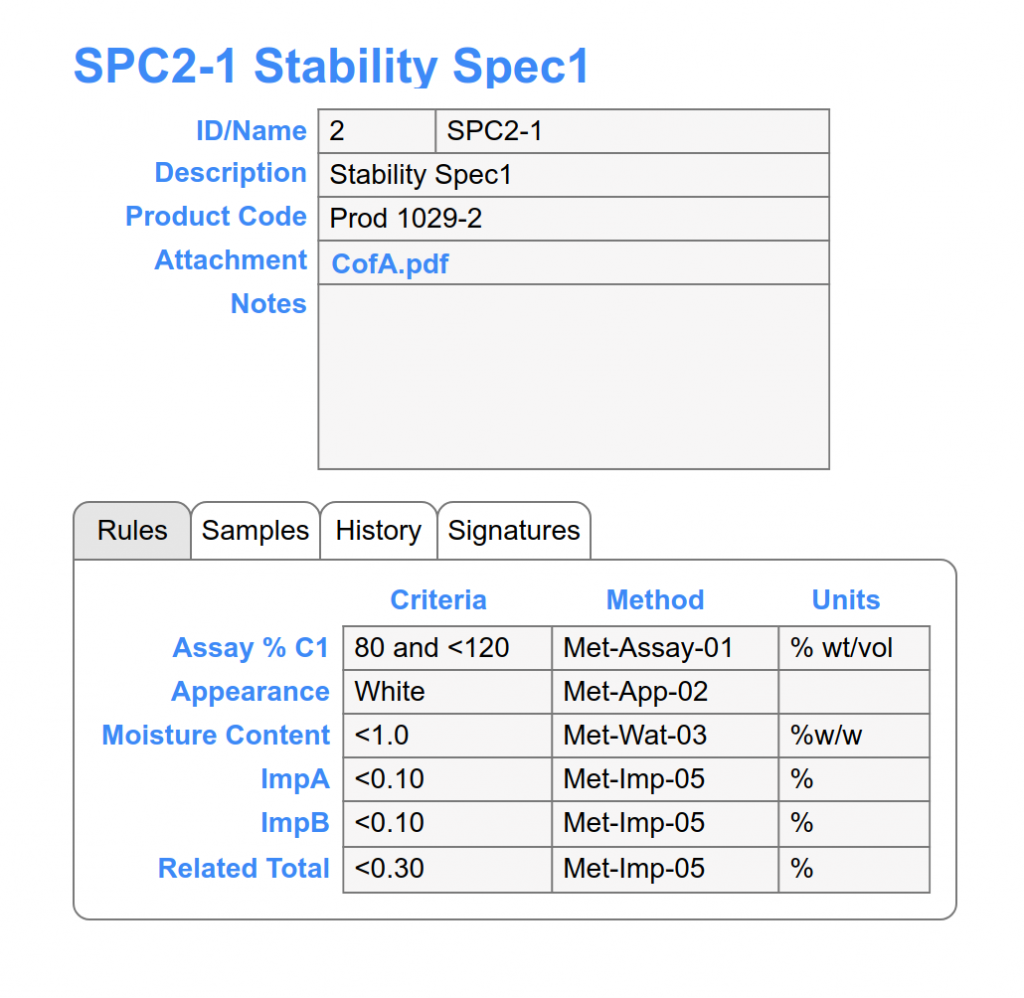
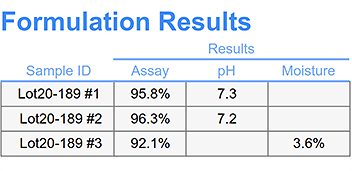
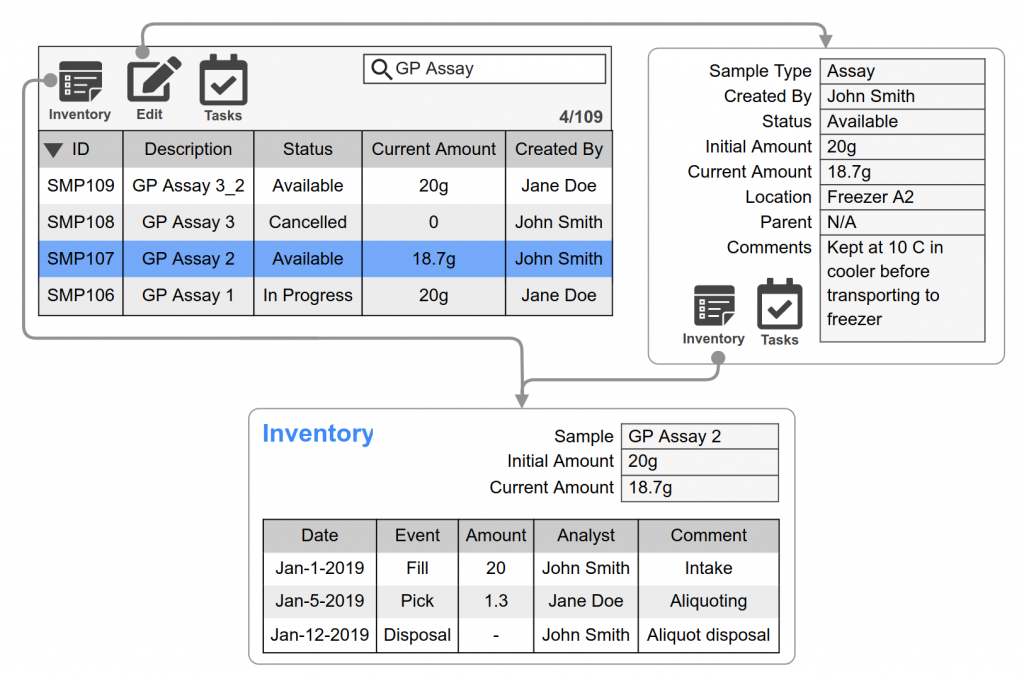
Comprehensive stability sample management including: location tracking, chain of custody, barcode scanning, inventory transaction scheduling an logs, and sample workflow with electronic signatures to capture status change.
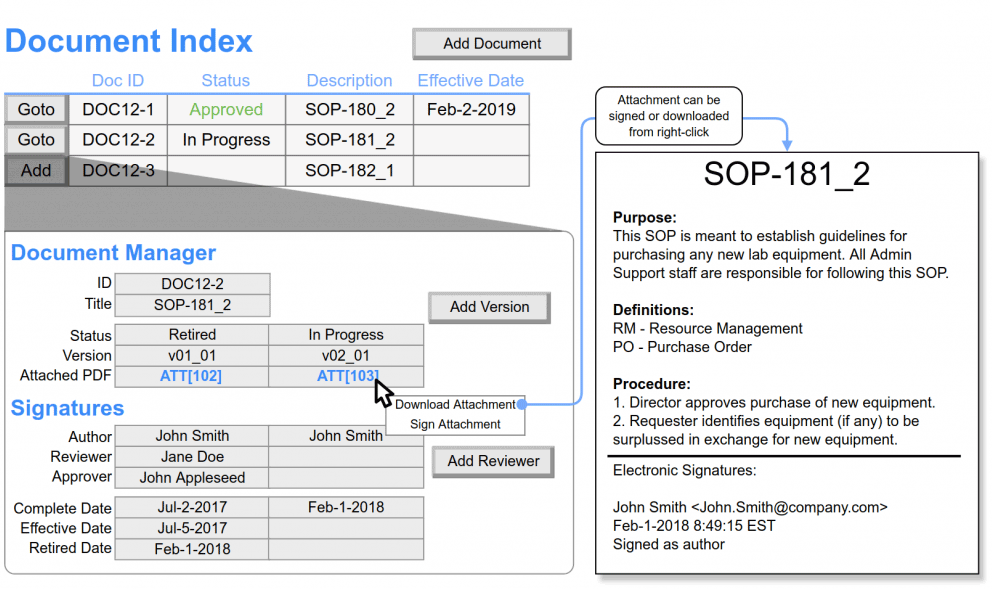
Document Repository
- Store and manage stability study-related documents, including protocols, reports, and certificates of analysis.
- Maintain version control for documents and ensures that the latest versions are used in stability studies.
Integration with Laboratory Instruments
- Instrument connectivity: Integrates with laboratory instruments for automated data capture, reducing manual data entry errors.
- Data import/export: Allows for seamless data import and export with various file formats and systems.
User Access and Security
Role-based access control: Provides role-based permissions to control access to different system functions and data.
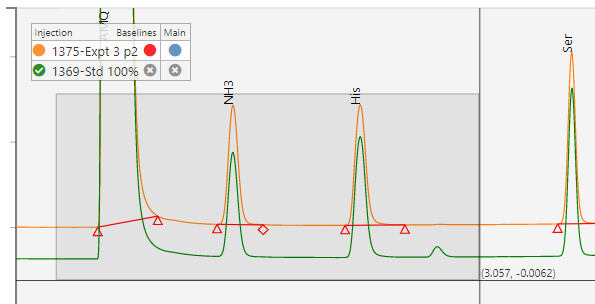
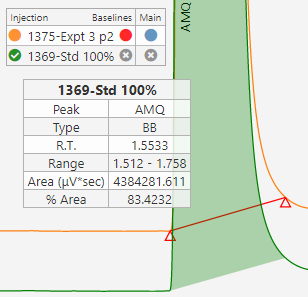
Sequence Visualization
A browser based sequence viewer eliminates the need for reviewers, approvers and decision makers to access & navigate the chromatography system.
A simple, demo version of SciCord’s SciChrom Viewer is accessible below. A guide is also available if you need more information about how SciChrom Viewer works.
SciChrom Viewer is meant to be used on a full desktop/laptop (not phone/tablet) and on the Chrome, Edge, or Firefox browsers. Please use one of these devices/browsers.
You can also download the user guide if you want to get an idea of how SciChrom Viewer works but don’t have access to a computer.
If you want a more technical discussion about stability in general, you can
read more here.