Chemical Handling & Storage
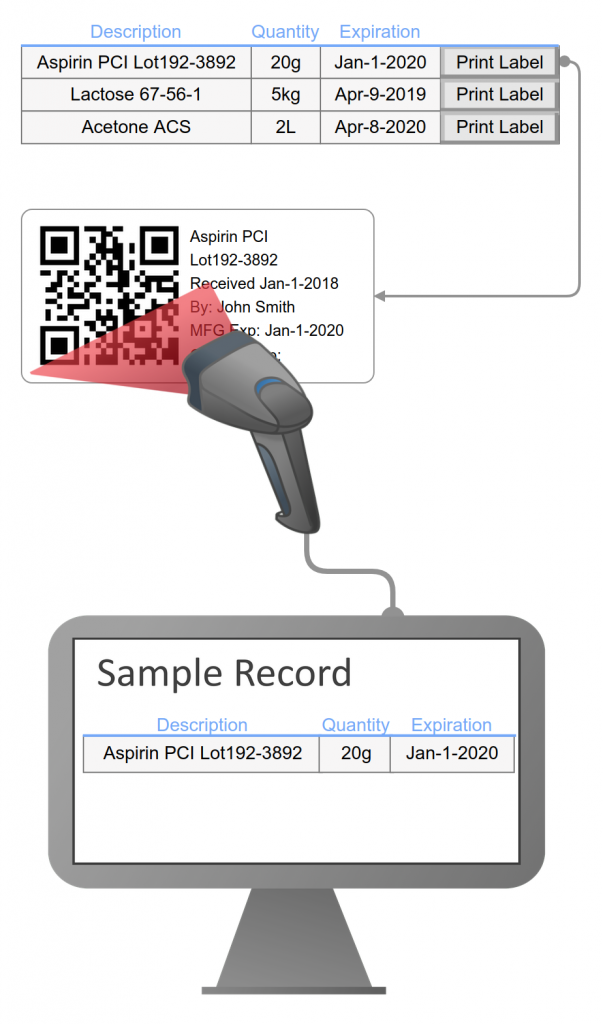
Attributes to record handling and storage requirements may be based on defined lists for consistency. Labels can include handling information to avoid contamination, degradation, or alteration. Labels can include storage information to ensure storage at specific temperature ranges and humidity levels to prevent deterioration.
Inventory Management
Schedule and track sample/chemical inventory movements to assist scientists in quickly locating and retrieving samples/chemicals.
Locations are defined within a hierarchical configuration representing your physical site. Chemical location is defined when a chemical is logged in and may be changed at any point in the chemical life. Location changes are tracked in the inventory log.
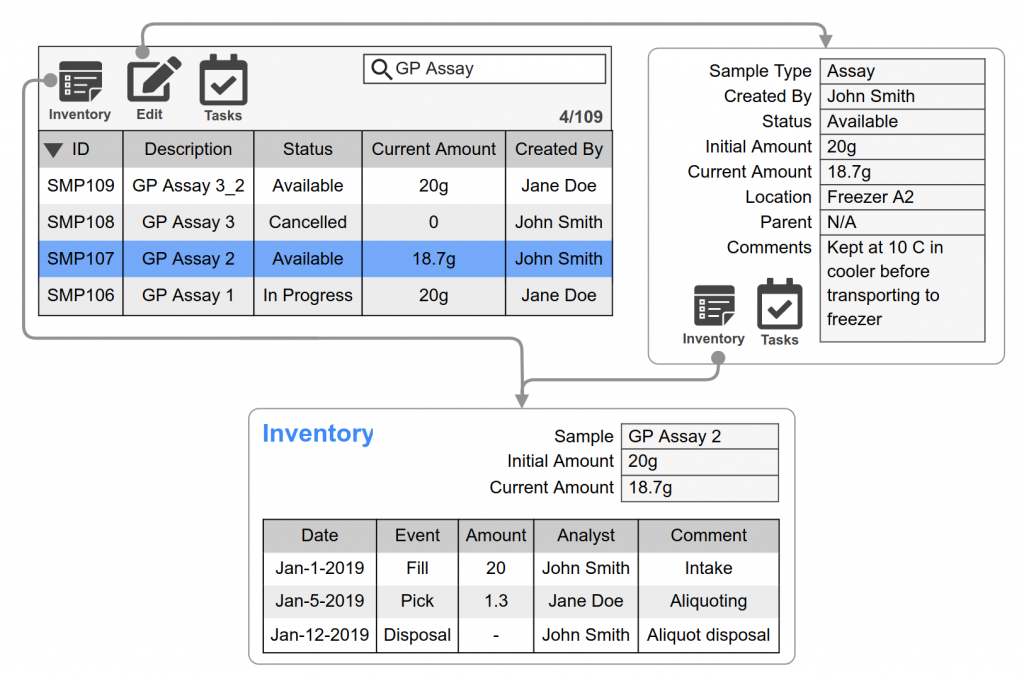
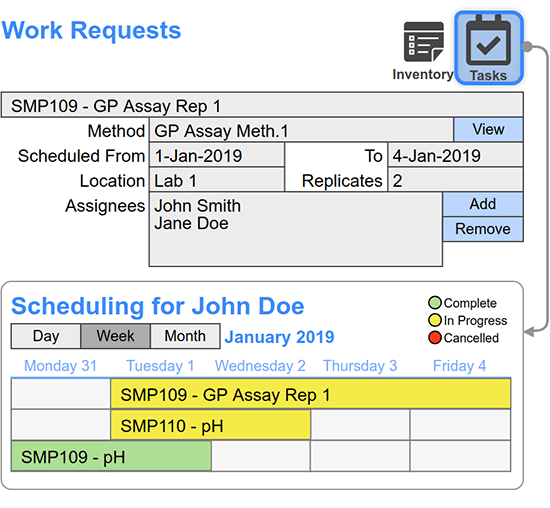
Scientists request or schedule inventory movements including how much of a chemical is needed, who needs it, and when.
Material managers fulfill requests or scheduled inventory movements. Inventory transactions are logged and may be electronically signed to fulfill chain of custody requirements. Amounts are automatically updated for each transaction.
Real-time tracking capabilities, allow the status and movement of chemicals to be monitored throughout their lifecycle.
are maintained and accessible, sample collection, storage conditions, usage, and disposal. This documentation is crucial for quality control and regulatory compliance.
Role based permissions
restrict access to approved sample managers to maintain sample security and confidentiality.