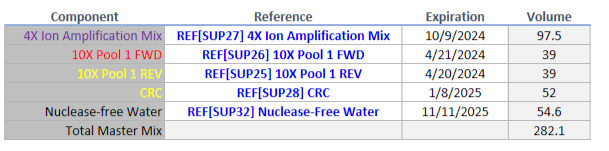
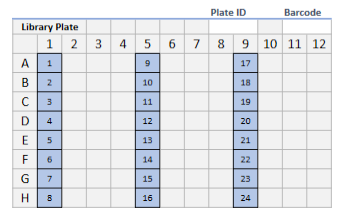
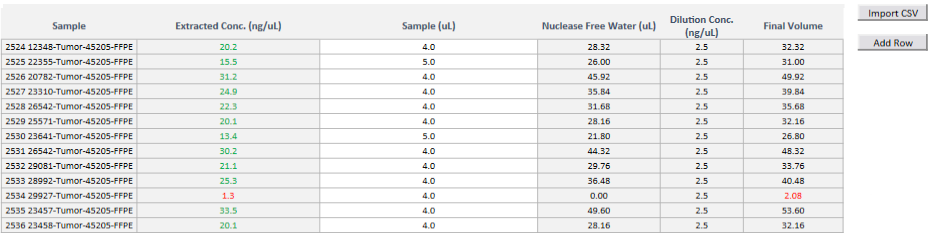
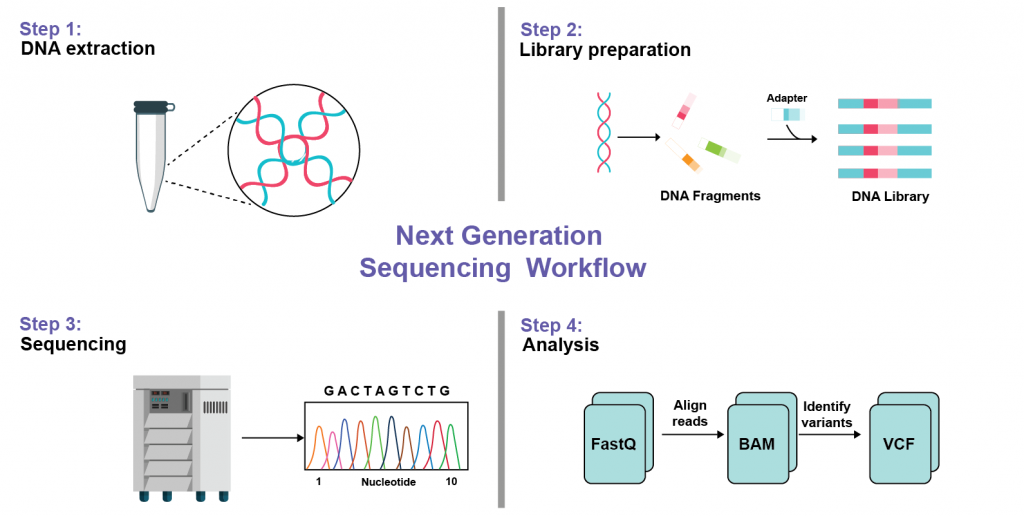
Platform and Features
Read through our full list of core features and platform capabilities
Solutions
Looking for something more specific? Read through the other solutions we support out of the box
Resources
Browse our archive case studies, functional documentation, and announcements.Looking for something specific? Search our site: