Are you currently managing formulation processes using disparate tools and manual methods, facing challenges in data organization, accuracy, and collaboration across your organization?
Discover a more streamlined approach to formulation management with the SciCord Formulation solution. Simplify your workflows, enhance data accuracy, and foster efficient collaboration. Unify your formulation processes with a single, integrated solution.
Efficient Configuration: The Definitions page enables defining key aspects of your formulation process, such as lot number prefixes, with easy linkage to the Samples page where unique identifiers are generated.
Resource Management: Effortlessly link and document the balances and equipment used during formulations, ensuring accurate resource tracking.
Enhanced Organization: Annotate your work with attributes such as project, study, or status (active/inactive) to easily locate previous work throughout the organization.
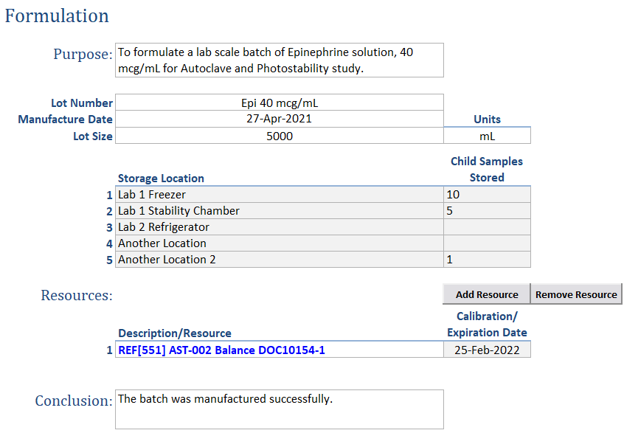
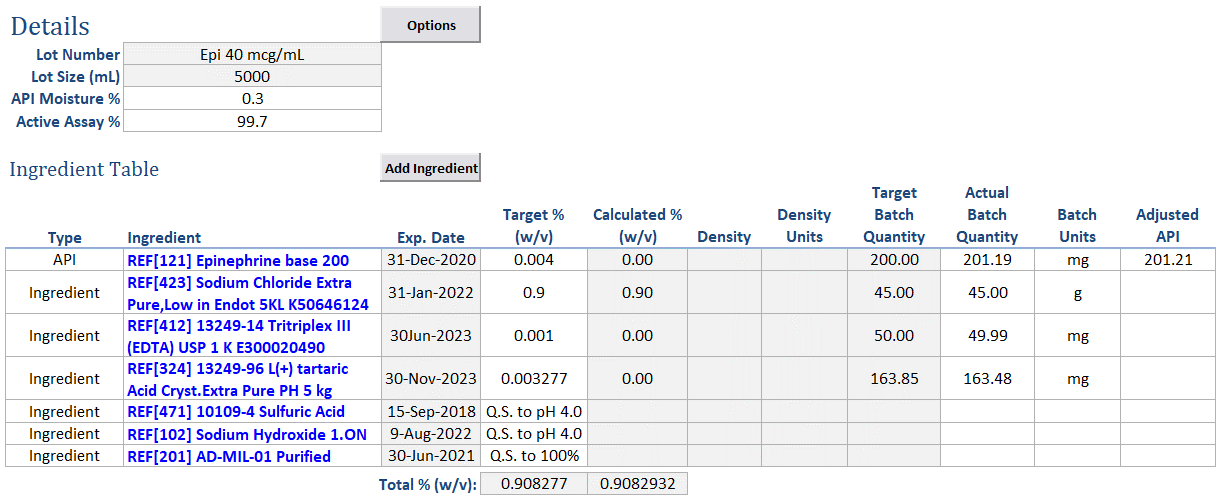
Streamlined Data Entry: Record references to ingredients with a single click, automatically populating essential information like physical characteristics, safety, and hazard details.
Precise Formulation: Define target amounts in the ingredient table, displaying target weights or volumes based on purity and other factors to ensure accurate formulation.
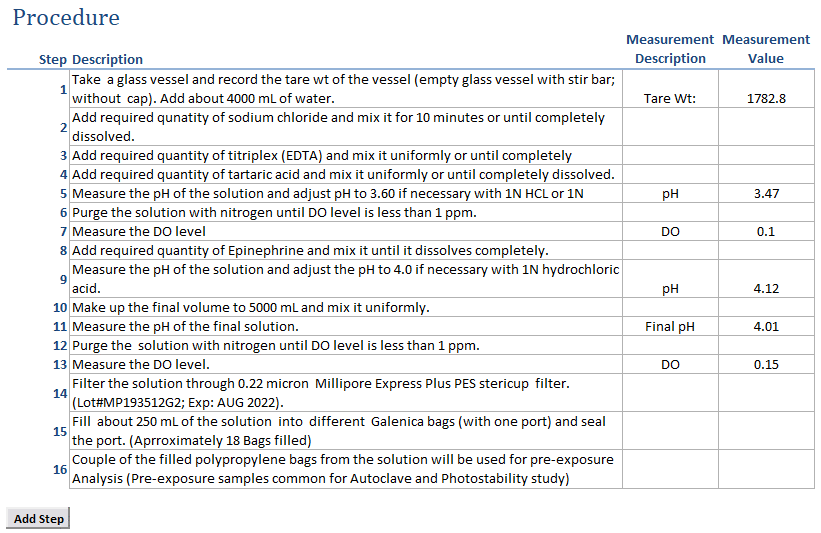
Intuitive Process Capture : Capture your intended process with ease, recording measurements as your formulation progresses.
Time Savings: Clone previous work, modify as needed, and execute, saving valuable time and ensuring consistency in your formulation processes.
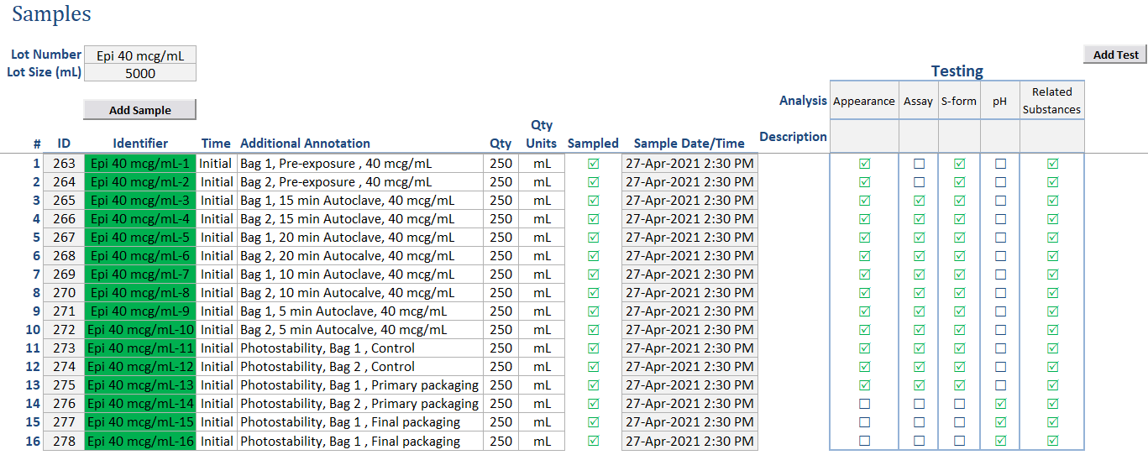
Streamlined Sample Creation: Easily create samples within the formulation template, assigning various tests and default attributes like quantity. These samples can be seamlessly used in other documents within the system, enabling data sharing and integration.
Holistic Sample Tracking: Define and label in-process samples, specifying required analyses. Monitor in-process test status within the context of your experiment.
Simplified Testing: Sample pulls and analyses can be automatically converted into work requests. These requests can be assigned to analysts for completion, fostering efficient collaboration and task allocation.
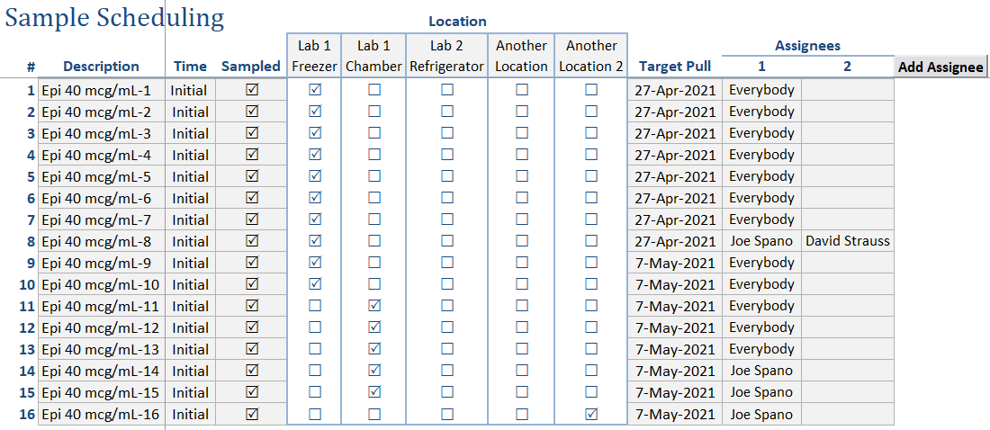
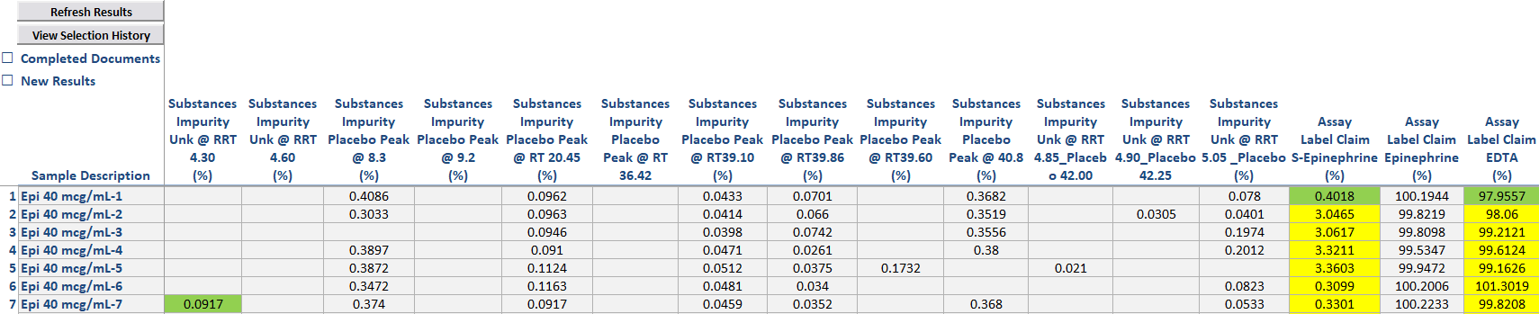
Centralized Results: As work requests are completed, their results can be seamlessly imported back into the Formulations template, providing a comprehensive overview of sample testing across all documents.
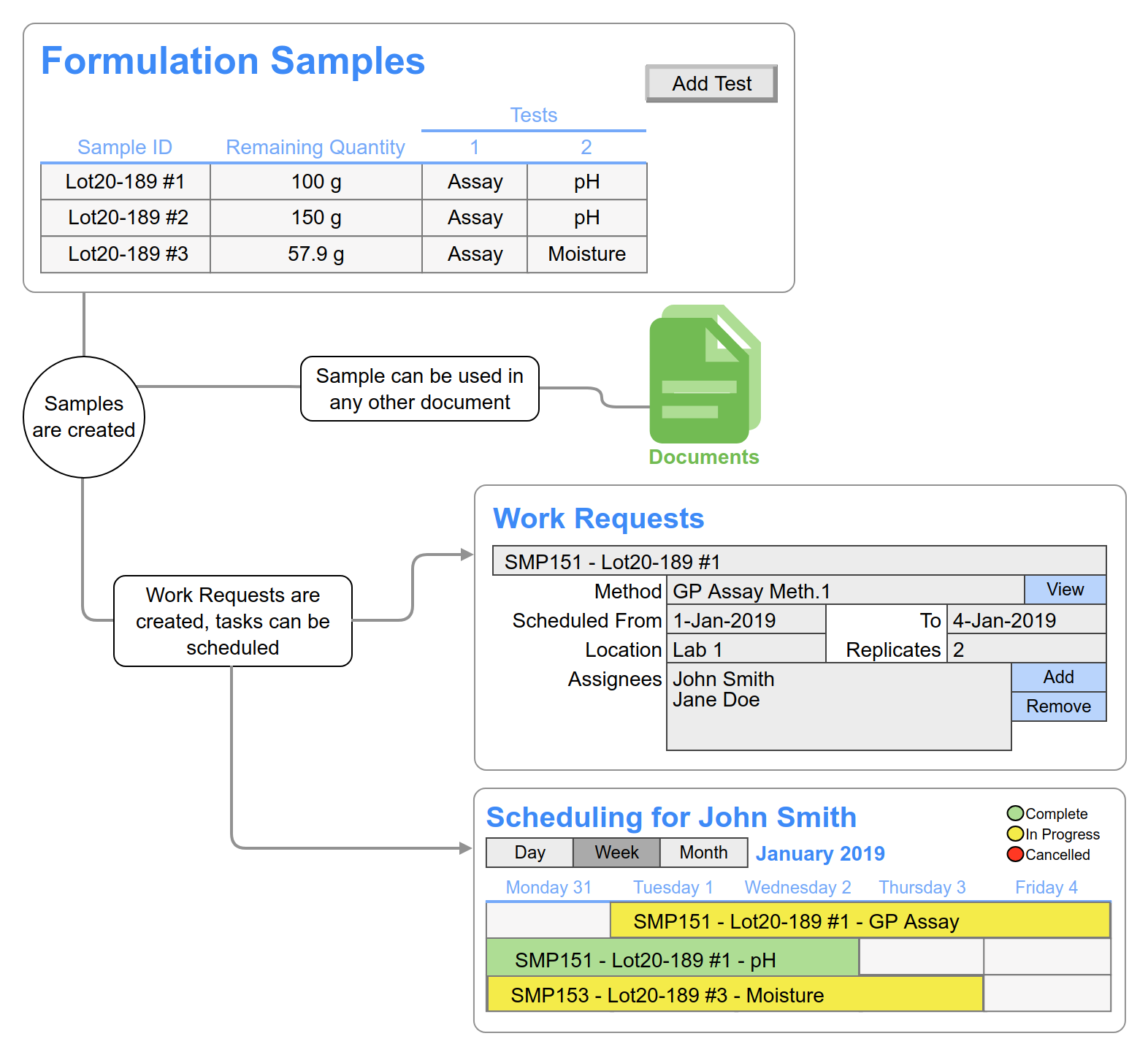
Automation: Benefit from built-in automation that eliminates the need for excessive manual inputs, such as the creation of samples, work requests, and inventory requests.
Reach out to Schedule a Meeting and get more information about how SciCord can fit into your lab
Don’t take our word for it.
We exceed our client’s demands everyday to make their research and discovery process simpler and more efficient.
This is by far the best value in science software (or anything else in science, really) that we’ve ever experienced. Other solutions in this price range had a fraction of the features, and those with the features cost 3x – 10x more. We’re very happy customers.

Josh Guyer,
Senior Pharmaceutical Scientist