Labeling
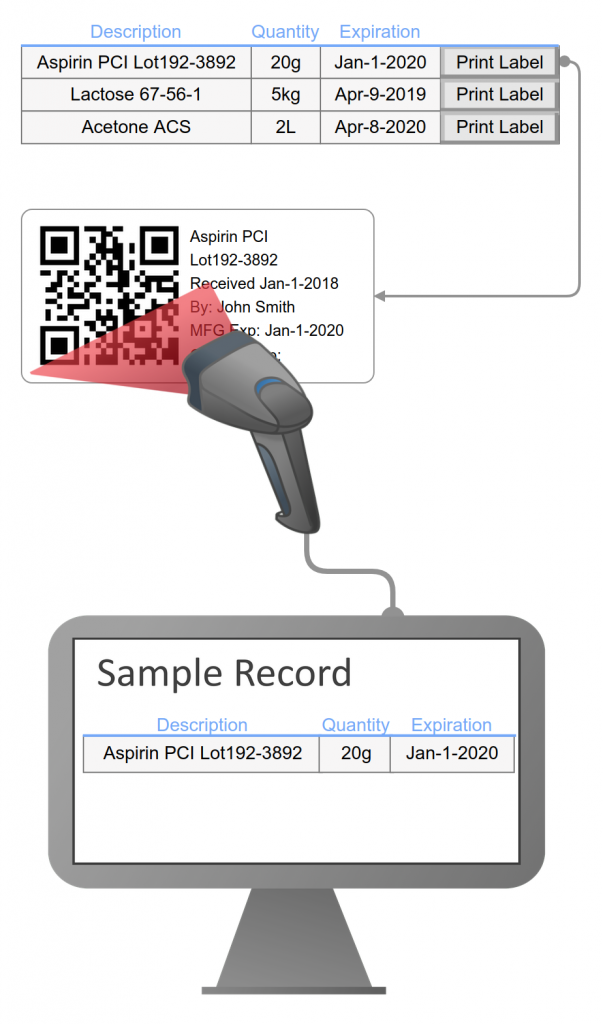
Labels including barcodes are generated for samples and laboratory resources such as equipment, solutions, standards, and chemicals. Label printing support for multiple labels per sample, custom prefix, and replicate numbers for each label (examples: vial 1 of 12, vial 2 of 12).
Sample Identification
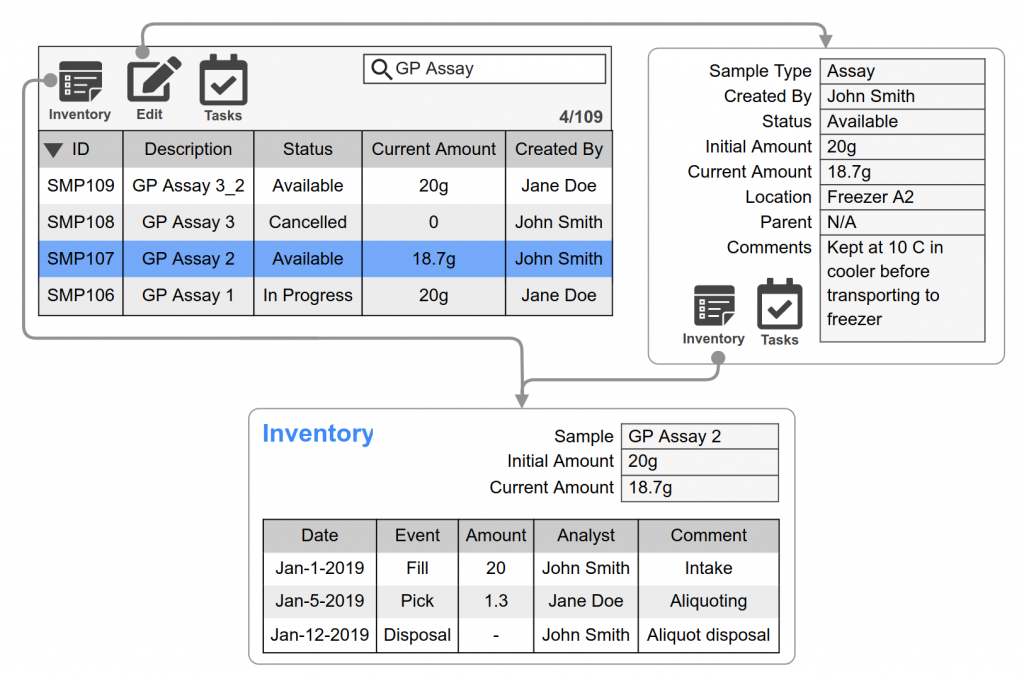
Samples are assigned unique identifiers with configurable prefixes for each sample type (examples: stability = STAB, Raw Material=RM). The numeric portion of the identifier increments from 1 for each sample type (examples: STAB1, RM23). Theses identifiers are used to clearly identify a specific sample and when interfacing with instruments or third-party applications. In addition, legacy or third-party sample identifiers can be recorded.