Reference Standard Logbook
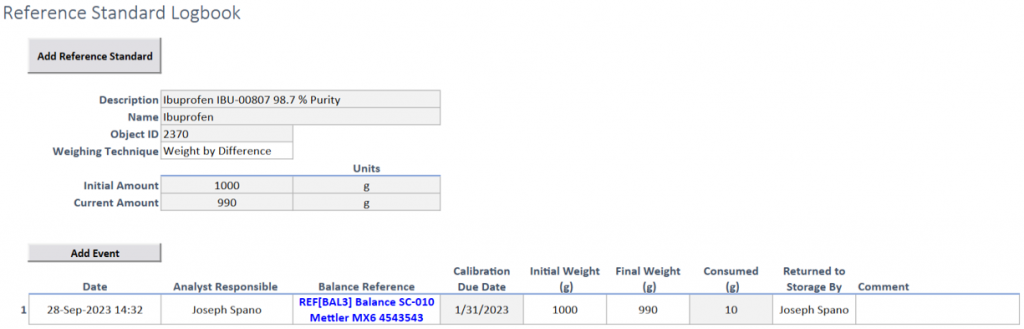
Efficient Logbook Management: Utilize a dedicated logbook for recording and managing reference standards, simplifying data organization.
Comprehensive Record Keeping: Record essential details such as descriptions, lots, expiration dates, and optional purity information upon receiving reference standards.
Reference Standard Integration
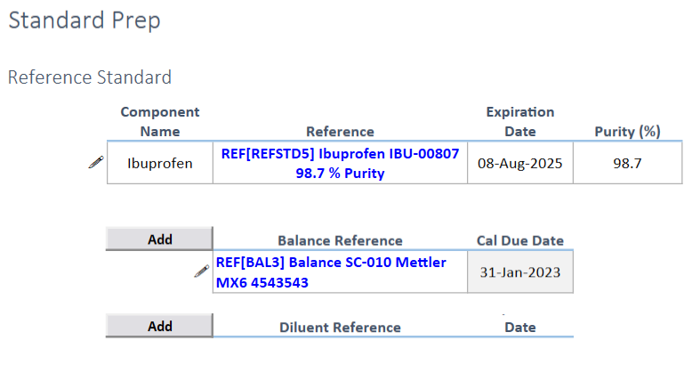
Seamless Integration: Incorporate reference standards into the standard preparation process, automatically identifying purity levels and reducing transcription errors.
References: Easily link and document any balances, equipment, or diluents used.
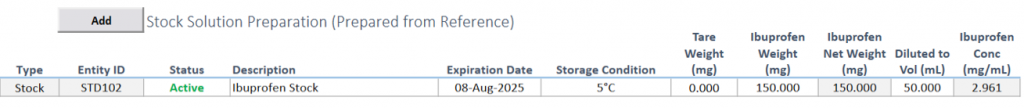
Stock Preparation
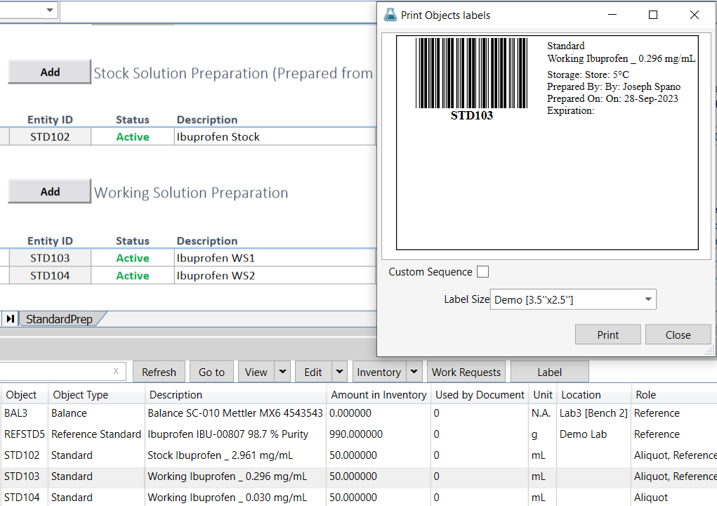
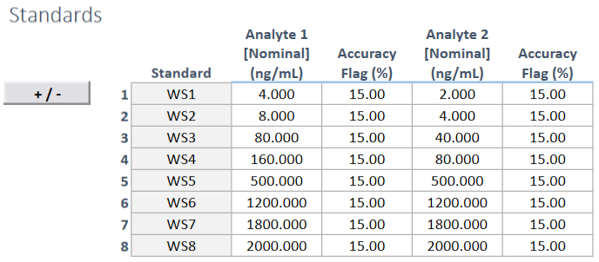
Effortless Recording: Document the preparation of stock, working, linearity, and quantitative limit (QL) standards with ease, ensuring complete data records.
Validated Formulas: Calculate standard concentrations automatically using validated formulae, guaranteeing precision.
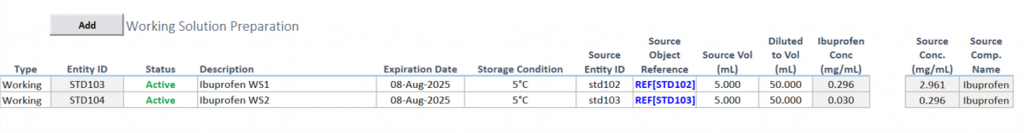
Working Standard Preparation
Real-time Accuracy: Record standard concentrations automatically at the point of use, guaranteeing real-time data accuracy and traceability.
Flexible Dilution Schemes: Document various dilution schemes for preparing working, limit, or other standards, enhancing process flexibility.
Multi Component Dilutions
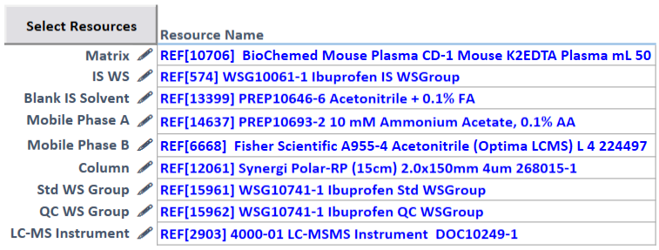
Multicomponent Dilution: Explore more complex dilution schemes involving multiple stock solutions.

Label Printing
Transcription Error Elimination: Automatically generate bar-coded labels for each standard, significantly reducing transcription errors during identification.
Clear Traceability: Ensure clear and traceable identification for precise record-keeping.
Usage Tracking in Batch Records
Comprehensive Records: Easily track the usage of each standard, including reference standards, stock solutions, and working solutions, providing a comprehensive history of their utilization.
Data Integration: Seamlessly associate each standard with analytical testing and concentrations used to generate sample results, enhancing data organization.