| Document Description |
Document Strategy |
| Validation Master Plan |
Establishes the objectives, policy, resources, execution and management of the SciCord ELN validation effort. |
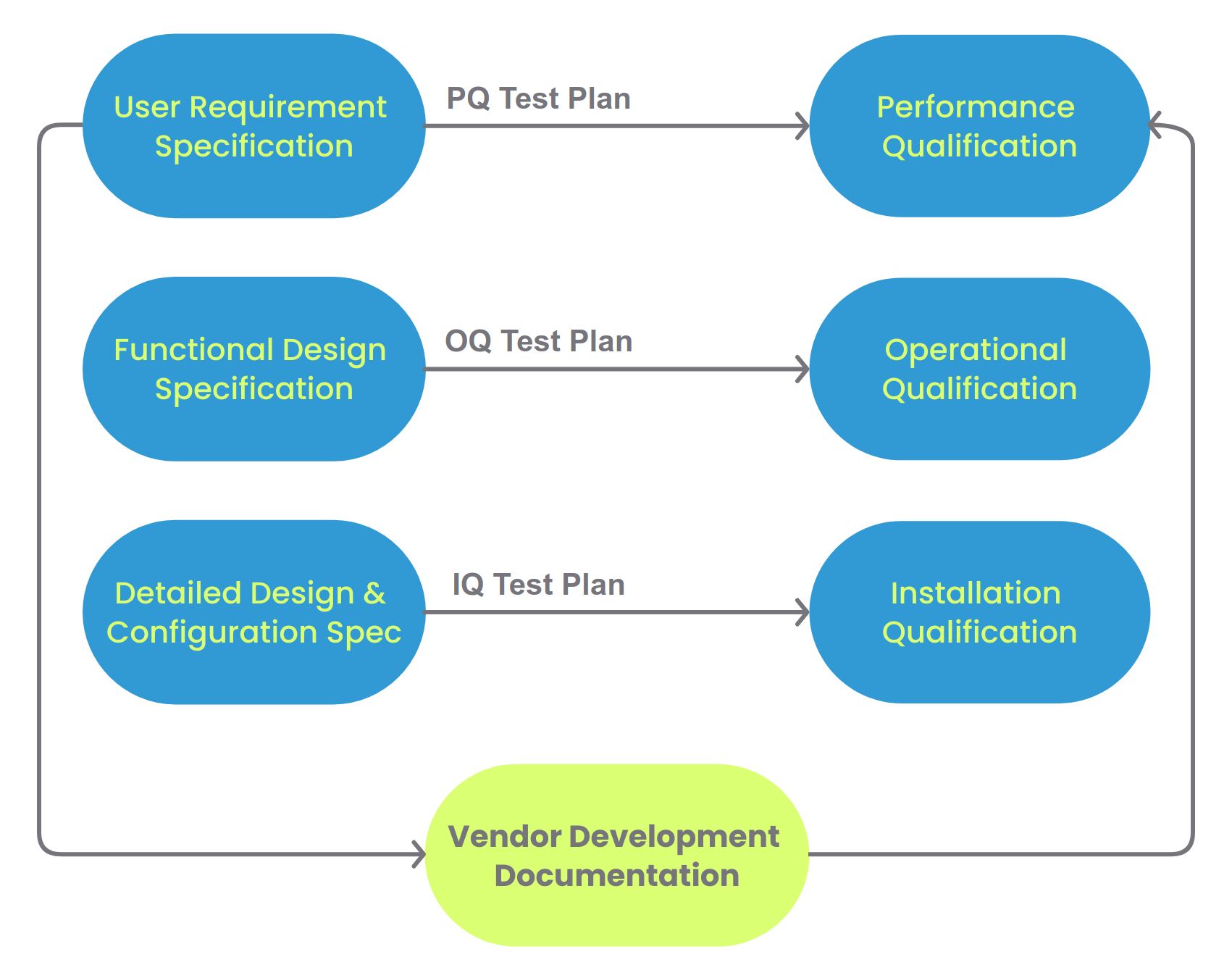
| User Requirements Specification for SciCord ELN |
User requirements defined in collaboration with Customer including review of Customer SOPs and work practices |
| Standard Operating Procedures |
Procedures for SciCord ELN Administration & SciCord ELN Use are written in collaboration with customer. |
| Training |
Training is provided to customer lead users. |
| Functional Specification for SciCord ELN |
SciCord Requirements Document and description of additional functional to be provided via configuration, templates, or reports. |
| Design Qualification for SciCord ELN |
Comparison of SciCord Functional Requirements to User Requirements Specification. Verifies that the proposed design and configuration of the system is suitable for its intended purpose. |
| Installation Qualification |
SciCord Production Environment IQ |
| Operational Qualification |
SciCord Test Plan and Summary Report. |
| Risk Assessment |
Identify risks associated with Customer deployment that will be mitigated procedurally or with PQ testing. |
| Vendor Audit |
Optional: Third party or customer Quality Assurance audit (1 day) at customer expense. |
| Performance Qualification |
Utilize the output of the risk assessment to design and execute test cases intended to verify system functionality as configured for use. |
| Validation Summary Report |
Validation report that provides an overview of activities. This report to also include a summary of associated validation documents, define the configuration of permissions associated with system roles, and provide a traceability matrix between requirements and associated testing. |
| System Release |
Document to be provided to Customer Quality Assurance to release SciCord ELN for production use. |





Recent Comments