Enhancing Lab Efficiency: A Comprehensive Informatics Platform
“
“The world of lab data software is not well-defined, but at least two types of systems that come up are ELNs and LIMSs . . . Despite how important LIMS are to biotech, I don’t know how a biotech is supposed to get one up and running . . . Unfortunately, I don’t know of any specific LIMS that works well for general use.”
– Brian Naughton, Boolean Biotech 2021
Mr. Naughton’s comments are insightful, primarily because he works in the biotech industry and acknowledges the vital role a LIMS (Laboratory Information Management System) plays. Even in the pharma and biotech industries, people are confused about the differences between an ELN (Electronic Lab Notebook) and a LIMS.
SciCord works to bring LIMS and ELN, and other common lab software, together into one informatics platform. Our customers see at least a 30% efficiency improvement. These improvements are delivered through a secure cloud infrastructure that allows all your data to be accessible across your organization.
Why Software as a Service?
SaaS minimizes your total cost of ownership. Maintenance and backups are included, minimizing IT overhead.
SciCord provisions required hardware, eliminating initial hardware purchase and refreshes.
Security
Award winning Azure cloud platform meets a broad set of international and industry-specific compliance standards, such as General Data Protection Regulation (GDPR), ISO 27001, HIPAA, FedRAMP, SOC 1 and SOC 2. Data is encryption at rest and in transit.
Backups and Replicates
Backups are made to two physical locations, Incremental Daily, Full Weekly, Full Monthly. Completed document bundles are provided to you for your own on-site storage.
Why Software as a Service?
SaaS also allows SciCord to utilize a more robust central cloud infrastructure, based on Microsoft Azure. The SaaS provider manages the application, provides hardware, maintains security, and routinely monitors performance. By contrast, a local-only implementation requires hardware to be managed on-site, dedicated staff to be assigned to maintain it, and increases the risk of data loss in the event of unforeseen accidents or hardware failures.
Implementing SciCord as a SaaS product also allows for quicker and smoother implementations. Typical benefits or advantages of a SaaS solution include:
- Reduced time to benefit
- Lower costs
- Scalability and integration
- New releases (upgrades)
- Easy to use and perform proof-of-concepts
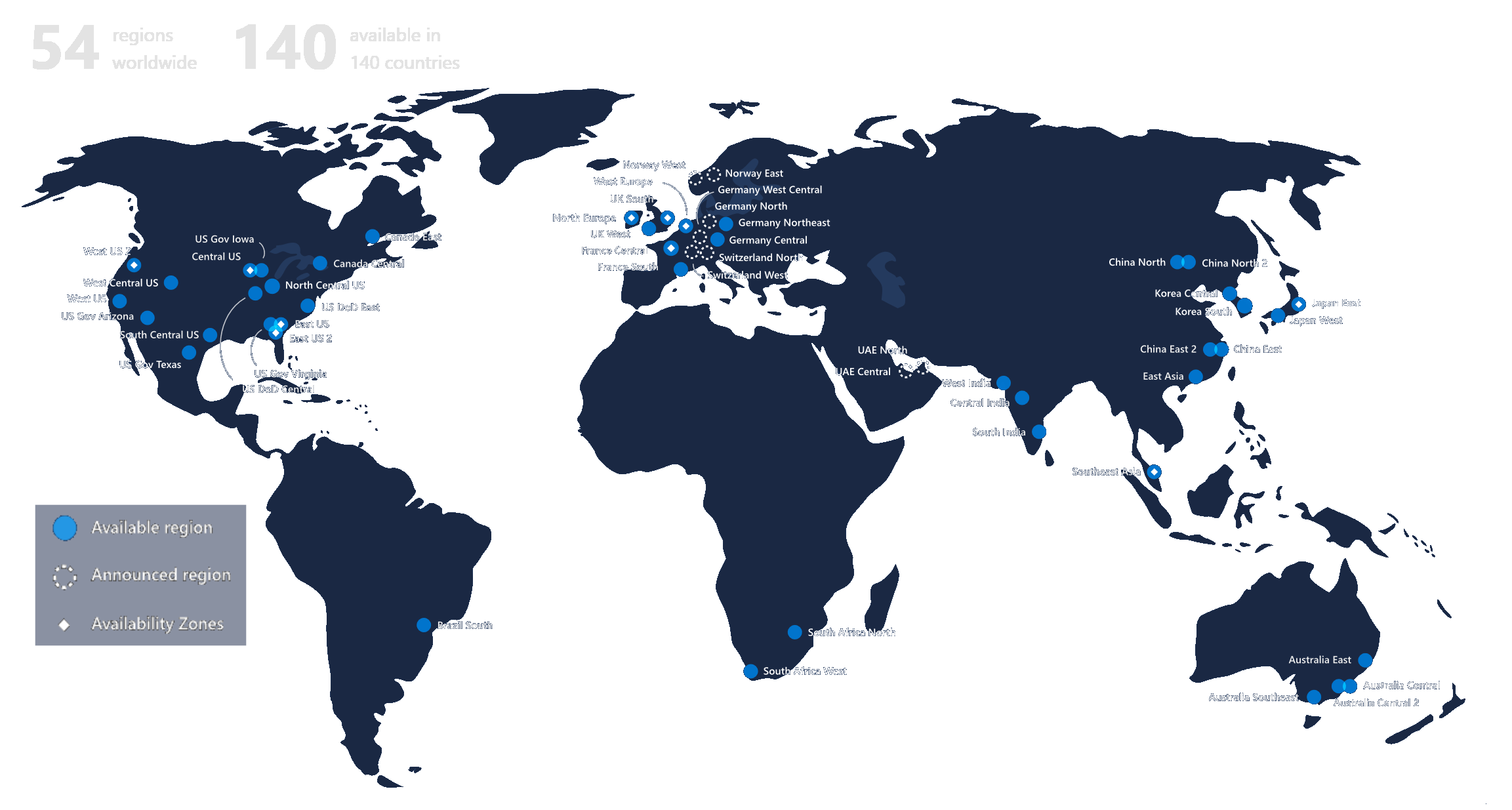
Azure
SciCord utilizes the Azure server network, making SciCord Informatics Platform accessible around the globe.
Third Party Integration
Web Services allow bidirectional exchange of information. This lets data be pulled from local-only or third-party repositories, such as an Empower installation, and upload it to the SciCord cloud for long-term storage and greater accessibility. Reviewers and managers can then view your data without direct access to the originating program or instrument.
Data can also be imported through database access, file transfers, etc.
ELN or LIMS?
SciCord’s SaaS approach also facilitates delivering multiple software solutions in one package. Laboratory Information Management Systems (LIMS) and Electronic Notebook (ELN) are key parts of this feature set.
- LIMS is focused on the management and tracking of laboratory samples, workflows, and associated data. Read more about us from a LIMS perspective here.
- ELNs are primarily designed to replace traditional paper laboratory notebooks. Read more about us from an ELN perspective here.
SciCord also includes features of document management (SDMS), batch record (EBR) or Manufacturing Execution System (MES), and Lab Execution (LES) systems.
SciCord in the Lab
The SciCord Informatics Platform was designed for the pharmaceutical industry and now includes an industry-leading application for labs developing inhaled products. SciCord provides the perfect platform for research organizations–such as biotech firms–that need a compliant documentation system from late Phase II through manufacture. In addition, the software is scalable and able to support global organizations as well as individual laboratories.
SciCord excels in Pharma labs, manufacturing, R&D, and biotech by:
Delivering Our Service Effectively
SciCord serves both GxP and non-GxP clients. SciCord provides the software and the infrastructure, maintenance, and support.
Protecting Data from External Threats
All SciCord systems are periodically updated with the current patches and security protocols. Data in the SciCord database is encrypted to protect it. Data is also encrypted when transmitted between a client’s site and the Cloud servers. Additionally, clients can expect external protection tests at least annually, with every major software release.
Protecting Data from Improper Internal Access and Vulnerability
A layered password system allows users to access only the information and resources required to perform their legitimate tasks. As designated by
FDA 21 CFR part 11, SciCord incorporates security features such as timestamps and digital signatures to assure regulated agencies that data on the system is consistent, not tampered with, and in general follows the data integrity directives mandated by the FDA. The system is also evaluated at least annually to verify its effectiveness.
Additionally, SciCord safeguards application and interface security by:
- Following SDLC (Software Development Life Cycle) guidelines, which outline phases of development, each with built-in control features.
- Using only in-house code developers.
- Penetration testing for each major version
- Regularly testing the software against OWASP Top 10 to identify any vulnerabilities.
Instrument Access
SciCord accommodates real-time data collection and displays from balances, pH meters, and other instruments by monitoring an IP address. For instruments that feature a result database, SciCord connects to the database, acquires the results, and records them. For all others, a printed output or created spreadsheet/CSV can be parsed for data import.
User Guidance
A lab doesn’t need to overflow with IT gurus or ‘superusers’ to reap the benefits SciCord provides. Users only need PCs that run Windows and basic working knowledge of spreadsheet software. SciCord provides initial instruction and training as well as periodic support.
SciCord offers the support required to implement and customize the software to fit a client’s needs. Each client is assigned a Project Manager to coordinate the deliverables between each lab’s personnel and the SciCord personnel.















Recent Comments