Are you grappling with the complexities of documenting your synthetic experiments? Do you find yourself spending excessive time managing data across various systems and struggling to maintain clear records? Are data accuracy, collaboration, and regulatory compliance challenges you face on a regular basis?
Discover a simpler and more efficient way to handle your synthetic chemistry experiments. The SciCord Synthetic Chemistry solution centralizes data management, streamlines documentation, and enhances collaboration. Seamlessly integrate your synthetic chemistry workflow to ensure precision, efficiency, and data traceability
Experiment Annotation: Easily annotate your experiment with attributes like project, route, and target, making your experiments searchable across all documents for efficient retrieval.
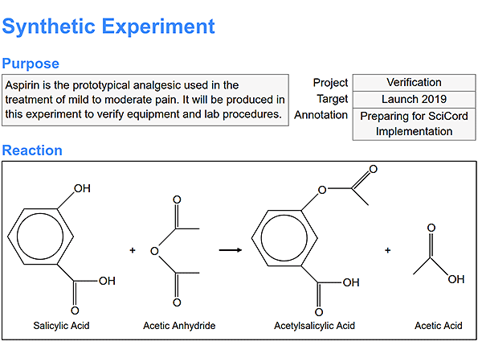
Purpose and Reaction Recording: Record the experiment’s purpose and reaction details using text descriptions or image insertions, ensuring comprehensive documentation.
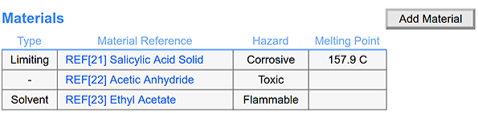
Materials Reference: Streamline materials management by referencing items in inventory with a single click. Automatically populate critical information such as physical characteristics, safety, and hazard data.
Seamless Material Access: Access original solution or standard preparation documents used for materials when clicking on references, enhancing traceability.
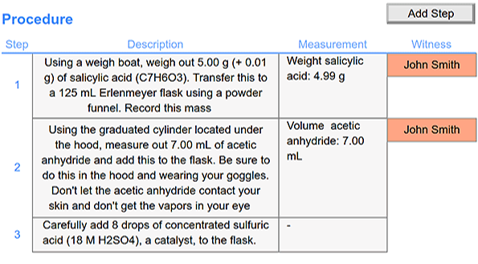
Procedure Recording: Document experiment procedures and note measurements or observations as the experiment progresses. Facilitate collaboration by enabling witness signatures for each step.
Efficient Workflow Replication: Clone previous work, modify it to suit your current needs, and execute experiments efficiently, ensuring consistency in your synthetic chemistry processes.
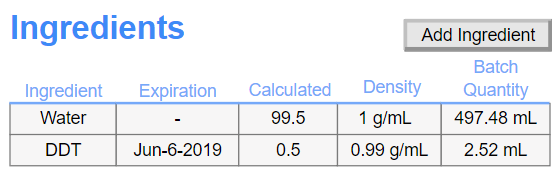
Customizable Materials Table: Define ingredient amounts and expected products with flexible materials tables adaptable to your organization’s workflow.
In-Process Sample Management: Define and label in-process samples, specifying required analyses. Easily monitor in-process test status and results within the context of your experiment.
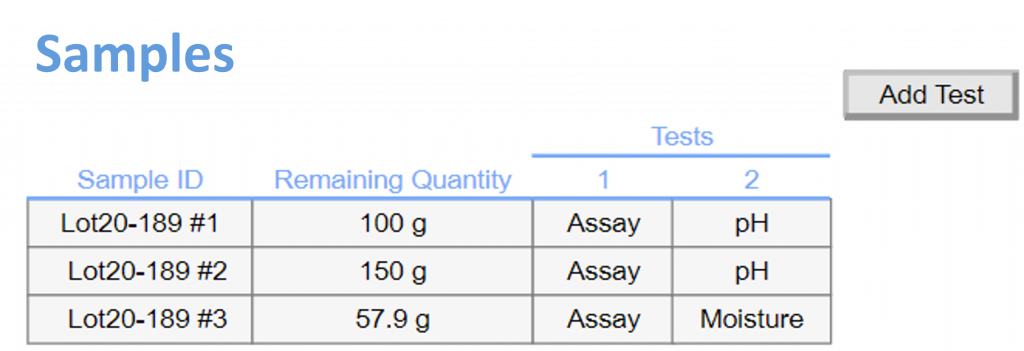
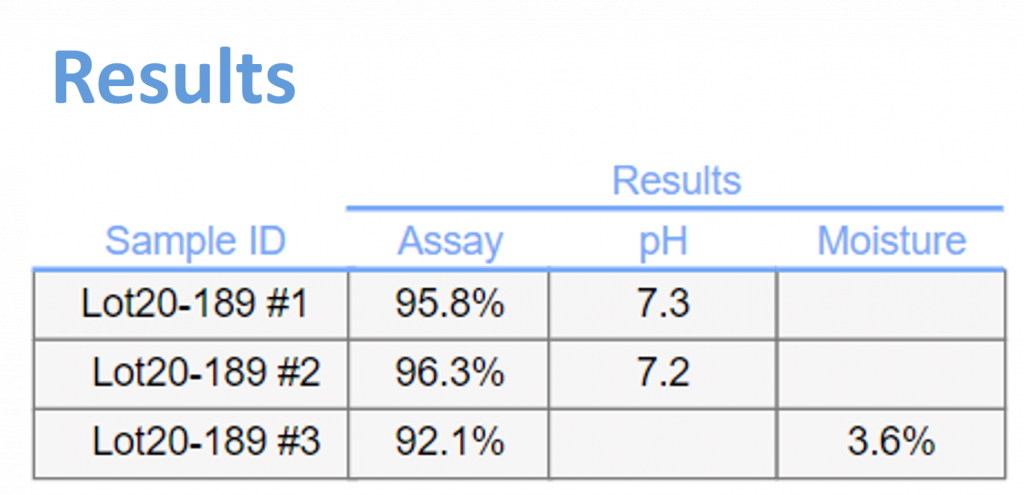
Centralized Results: As analyses are completed, their results can be seamlessly imported back into the Formulations template, providing a comprehensive overview of sample testing across all documents.
Reach out to Schedule a Meeting and get more information about how SciCord can fit into your lab
Don’t take our word for it.
We exceed our client’s demands everyday to make their research and discovery process simpler and more efficient.
This is by far the best value in science software (or anything else in science, really) that we’ve ever experienced. Other solutions in this price range had a fraction of the features, and those with the features cost 3x – 10x more. We’re very happy customers.

Josh Guyer,
Senior Pharmaceutical Scientist