Summary
SciCord Solution is provided as a Commercial off-the-shelf software or COTS. SciCord provides a complete package documenting control of the software product. Additionally, documentation and testing are required to verify the software product meets the organization requirements within the target environment with appropriate control of people, hardware, and process. SciCord’s pharmaceutical validation Service is intended to provide the required customer specific documentation.
Goal
Computer system validation of a COTS solution in the pharmaceutical industry must ensure an acceptable degree of documented evidence establishing confidence in the consistency, accuracy, and reliability against predetermined specifications.
The SciCord Validation Service goal is to ensure both technical and procedural controls are implemented for compliance with good documentation practices for electronic data generated by the system
Service Design
A Typical model for computerized systems validation is presented in the image below:
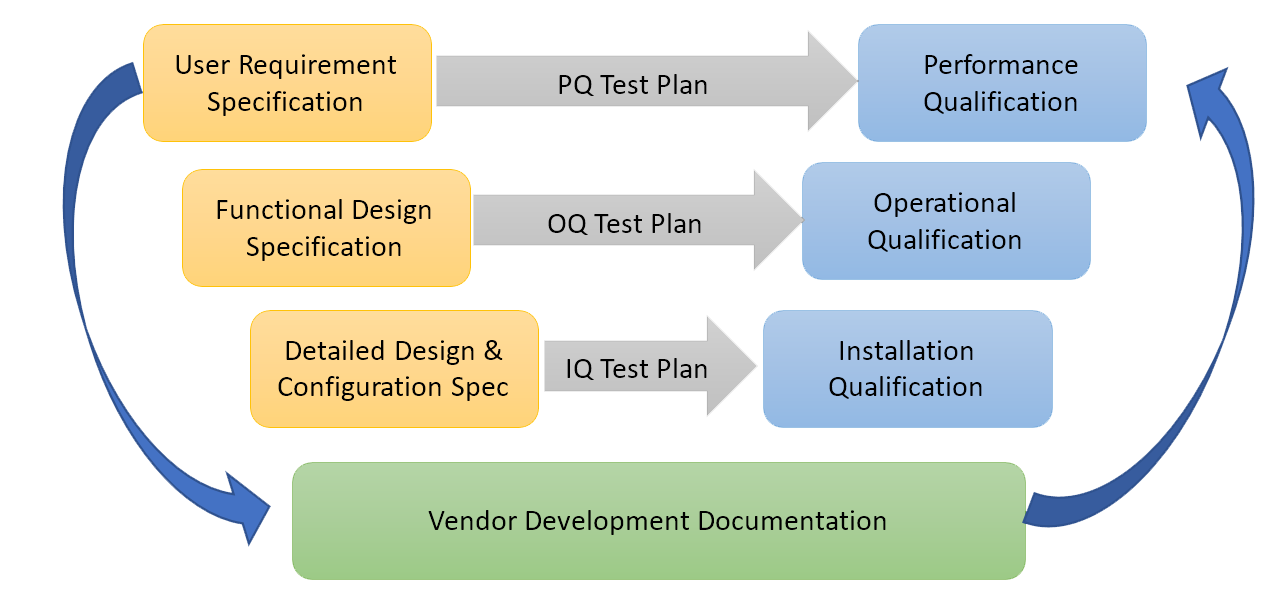
The Software as a Service (SaaS) delivery model of SciCord greatly simplifies the customer specific validation activities. Hardware in the SaaS delivery model is entirely the responsibility of the vendor, thereby removing a major complicating factor from the customer validation task list.For COTS solutions, much of the documentation/testing in the model above should be supplied by the vendor. The customer specific documentation includes User Requirements and Performance Qualification.
Deliverables
| Document Description |
Document Strategy |
| Validation Master Plan |
Establishes the objectives, policy, resources, execution and management of the SciCord ELN validation effort. |
| User Requirements Specification for SciCord ELN |
User requirements defined in collaboration with Customer including review of Customer SOPs and work practices |
| Standard Operating Procedures |
Procedures for SciCord ELN Administration & SciCord ELN Use are written in collaboration with customer. |
| Training |
Training is provided to customer lead users. |
| Functional Specification for SciCord ELN |
SciCord Requirements Document and description of additional functional to be provided via configuration, templates, or reports. |
| Design Qualification for SciCord ELN |
Comparison of SciCord Functional Requirements to User Requirements Specification. Verifies that the proposed design and configuration of the system is suitable for its intended purpose. |
| Installation Qualification |
SciCord Production Environment IQ |
| Operational Qualification |
SciCord Test Plan and Summary Report. |
| Risk Assessment |
Identify risks associated with Customer deployment that will be mitigated procedurally or with PQ testing. |
| Vendor Audit |
Optional: Third party or customer Quality Assurance audit (1 day) at customer expense. |
| Performance Qualification |
Utilize the output of the risk assessment to design and execute test cases intended to verify system functionality as configured for use. |
| Validation Summary Report |
Validation report that provides an overview of activities. This report to also include a summary of associated validation documents, define the configuration of permissions associated with system roles, and provide a traceability matrix between requirements and associated testing. |
| System Release |
Document to be provided to Customer Quality Assurance to release SciCord ELN for production use. |
Javscript required - please enable, or email us at info@scicord.com directly.

